How much of a temperature change is required to bring liquid magnesium from solid-liquid equilibrium to vaporliquid
Question:
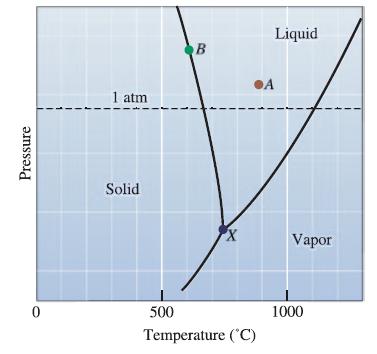
How much of a temperature change is required to bring liquid magnesium from solid-liquid equilibrium to vaporliquid equilibrium at 1 atmosphere?
Transcribed Image Text:
Pressure 0 1 atm Solid B X 500 Temperature (°C) Liquid A Vapor 1000
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
Temperature change Power Tempe...View the full answer

Answered By

Willis Omondi
Hi, I'm Willis Omondi, a proficient and professional academic writer. I have been providing high-quality content that best suits my clients and completing their work within the deadline. All my work has been 100% plagiarism-free, according to research from my services, especially in arts subjects and many others
0.00
0 Reviews
10+ Question Solved
Related Book For 

The Science And Engineering Of Materials
ISBN: 9781305076761
7th Edition
Authors: Donald R. Askeland, Wendelin J. Wright
Question Posted:
Students also viewed these Engineering questions
-
How should a monopsonist decide how much of a product to buy? Will it buy more or less than a competitive buyer? Explain briefly.
-
How much of a price premium do you think national brands ought to command over private brands? Justify your position.
-
A government is trying to decide how much of a public good to provide. The willingness-to-pay curves for each of its two citizens are as given in the diagram. The marginal cost curve for the public...
-
You are the manager of a local coffee shop. There are two types of customers in your market, coffee addicts, and casual drinkers. Because coffee addicts buy large amounts of coffee, they are more...
-
Starting with the exchange rate of R = $2/1, draw a figure showing the exchange rate under a crawling peg system with the nation appreciating its currency by 1 percent at the end of each month for...
-
Four methods of achieving an input impedance greater than 100 kΩ are shown in the ac schematics of Fig. 11.50. (a) Neglecting flicker noise and capacitive effects, derive expressions for...
-
An art gallery purchases a painting for $11,400 on terms FOB shipping point. Additional costs in obtaining and offering the artwork for sale include $130 for transportation-in, $150 for import...
-
Briefly describe some of the similarities and differences between GAAP and IFRS with respect to the accounting for inventories.
-
In 2020, Staged Home Ltd. completed the following transactions involving delivery trucks: July 5 Traded in an old truck and paid $25,600 in cash for furniture. The accounting records on July 1 showed...
-
Sandra?s Purse Boutique has the following transactions related to its top-selling Gucci purse for the month of October. Required: 1. Calculate ending inventory and cost of goods sold at October 31,...
-
How can solid solutions form in ceramic systems?
-
Do we need 100% solid solubility to form a solid solution of one material in another?
-
The following option prices were observed for calls and puts on a stock on July 6 of a particular year. Use this information for problems. The stock was priced at 165.13. The expirations are July 17,...
-
Company, a soft-drink vendor, has created a table of costs for three stocking decisions for three different states of nature: Alternatives States of Nature Low Demand Medium Demand High Demand Large...
-
utilizes a project scheduling software application to develop a project schedule for a construction project.
-
Kimi is a server or restaurant and relies on tips from a customers to make a living. She doesn't really enjoy her job and frequently think about quitting because she is constantly having 2% a happy...
-
What represents revenues (Inflow) and expenses (Outflow) for a healthcare organization? Your paper should include information on sources of healthcare revenue (governmental and private payers), how...
-
case study analysis should be a thoughtful write up including: 1.a brief case analysis 2.key questions and answers from the case from your unique perspective 3.a summary and/or recommendations...
-
True or False. Work is a physical example of a vector.
-
Explain the term "Equivalent Units". Why are they calculated in process costing? [4 Marks] [minimum 350 words]
-
Which of the following statement(s) is(are) true? a. A radioactive nuclide that decays from 2.00 10 21 atoms to 5.0 10 20 atoms in 16 minutes has a half-life of 8.0 minutes. b. Nuclides with large...
-
A certain radioactive nuclide has a half-life of 3.00 hours. a. Calculate the rate constant in s -1 for this nuclide. b. Calculate the decay rate in decays/s for 1.000 mole of this nuclide.
-
Complete the following table with the nuclear particle that is produced in each nuclear reaction. Initial Nuclide 23 Pu 94 214Pb 82 60, 27 99- 43 Tc 93Np Product Nuclide 2351 92 214p 60 Ni 28 44Ru...
-
Marie Forleo, a marketing trainer and host of MarieTV, presents the eight tips for genuine networking. Do you agree or disagree with her suggestions? Discuss how this information is useful to you and...
-
Identify all relevant costs or revenue that are applicable to production- constrained decisions 1. Contributions margin of product 2. Interference with other production 3. Contribution margin per...
-
Gammaro Compary manufactures wallets from fabric. In 2 0 1 9 , Gammaro made 2 , 1 5 0 , 0 0 0 wallets using 1 , 2 5 0 , 0 0 0 yards of fabric. In 2 0 1 9 , Gammaro has capacity to make 2 , 8 0 0 , 0...

Study smarter with the SolutionInn App


