In the presence of nitric acid, UO 2+ undergoes a redox process. It is converted to UO
Question:
In the presence of nitric acid, UO2+ undergoes a redox process. It is converted to UO22+ and nitric oxide (NO) gas is produced according to the following unbalanced equation:
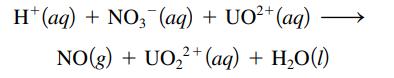
If 2.55 × 102 mL NO(g) is isolated at 29οC and 1.5 atm, what amount (moles) of UO2+ was used in the reaction?
Transcribed Image Text:
H+ (aq) + NO3(aq) + UO²+ (aq) NO(g) + UO₂²+ (aq) + H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
Amount moles of ...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
If 2-methylpropane is brominated at 125C in the presence of light, what percent of the product will be 2-bromo-2-methylpropane? Compare your answer with the percent given in Problem 4 for...
-
In the presence of the enzyme aconitase, the double bond of aconitic acid undergoes hydration. The reaction is reversible, and the following equilibrium is established:
-
At 500 K in the presence of a copper surface, ethanol decomposes according to the equation C2H5OH(g) CH3CHO(g) + H2(g) The pressure of C2H5OH was measured as a function of time, and the following...
-
Why do we need Normalization? How many forms of Normalization exist? What is the minimum normal form required? Describe each normal form List Normalization conversion process
-
In Shelanu v. Three Print, the Ontario Court of Appeal held that franchisors owe a duty of good faith to franchisees. What factors led the court to this conclusion? In what other contractual...
-
Leah Wells, a yoga instructor, started a company that sells athletic yoga clothing. You are Leahs marketing manager. Round your answers to two decimal places. a. After assessing the competitors, you...
-
What were the outcomes and how did they compare with the objectives?
-
An airline with operations in San Diego, California, must staff its ticket counters inside the airport. Ticket attendants work 6-hour shifts at the counter. There are two types of agents: those who...
-
Due to erratic sales of its sole product-a high-capacity battery for laptop computers-PEM, Incorporated, has been experiencing financial difficulty for some time. The company's contribution format...
-
Investing involves making informed decisions, which means researching companies and industries before plunking down your hard-earned money! An excellent source of information about a company is the...
-
We state that the ideal gas law tends to hold best at low pressures and high temperatures. Show how the van der Waals equation simplifies to the ideal gas law under these conditions.
-
A large flask with a volume of 936 mL is evacuated and found to have a mass of 134.66 g. It is then filled to a pressure of 0.967 atm at 31 C with a gas of unknown molar mass and then reweighed to...
-
Sonix Electronics is a dealer of industrial refrigerator. Its average selling price of an industrial refrigerator is $5,000, which it purchases from the manufacturer for $4,200. Each month, Sonix...
-
1. The following data are available for JURIS DOCTOR CORP: Purchased raw materials from supplier amounting to P 40,000 on account.; During the month, raw materials costing P 30,000 were issued to...
-
The following financial information is available for Concord Corporation. (in millions) 2025 2024 Average common stockholders' equity $2,500 $2,600 Dividends declared for common stockholders 305 594...
-
Vecton's Bakery manufactures apple turnovers that passes through 4 sequential processes. Production data for February for Department 4 of the operation is as follows: Production data Units Opening...
-
write a code in java where we apply the sets and subsets to obtain functions as results. Let A= {1,2,3,4}, B={5,6,7,0}, C={8,9,10,11} and f: AB g:BC h: BC, all function are 1 to 1 a) Form the...
-
Consider the following LC-3 program. .ORIG x3000 LEA R1, LABEL LDR RO, R1, #231 LDI R1, LOCAL AND R3, R3, #0 LOOP AND R2, RO, R1 BRZ SHIFT ADD R3, R3, #1 SHIFT ADD R1, R1, R1 BRnp LOOP HALT LABEL...
-
Find the value of each determinant. |1 3 -2 1 -5 6 1 2 3|
-
Floyd Distributors, Inc., provides a variety of auto parts to small local garages. Floyd purchases parts from manufacturers according to the EOQ model and then ships the parts from a regional...
-
A compound with molecular formula C 10 H 10 O 4 produces a 1 H NMR spectrum that exhibits only two signals, both singlets. One signal appears at 3.9 ppm with a relative integration value of 79. The...
-
For each of the following compounds, predict the number of signals and location of each signal in a 13 C NMR spectrum: (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) H.
-
Compare the following two constitutional isomers. The 13 C NMR spectrum of the first compound exhibits five signals, while the second compound exhibits six signals. Explain. .
-
You are thinking of buying a stock priced at $99 per share. Assume that the risk-free rate is about 4.5% and the market risk premium is 6.4%. If you think the stock will rise to $125 per share by the...
-
The transactions in this practice set were completed by Hydro Paddle Boards, Inc. during January, the first month of the companys fiscal year. Hydro Paddle Boards, Inc. is a manufacturing corporation...
-
Al preparar el estado de resultados pro forma, cules de las siguientes partidas se deducen de las utilidades brutas para llegar a las ganancias despus de impuestos? Pregunta de seleccin mltiple....

Study smarter with the SolutionInn App


