Sodium chloride is added to water (at 25 C) until it is saturated. Calculate the Cl
Question:
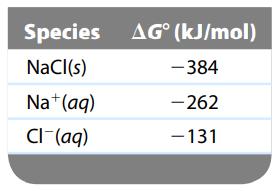
Sodium chloride is added to water (at 25οC) until it is saturated. Calculate the Cl- concentration in such a solution.
Transcribed Image Text:
Species NaCl(s) Na + (aq) Cl- (aq) AG (kJ/mol) -384 -262 - 131
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
The Cl concentration in such a solution ...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Lead chloride at first precipitates when sodium chloride is added to a solution of lead nitrate. Later, when the solution is made more concentrated in chloride ion, the precipitate dissolves. Explain...
-
A soluble salt, MX2, is added to water in a beaker. The equation for the dissolving of the salt is: MX2(s) M2+(aq) + 2X(aq); H > 0
-
Liquid water at 25 C and 1 bar fills a rigid vessel. If heat is added to the water until its temperature reaches 50 C, what pressure is developed? The average value of ( between 25 and 50oC is 36.2 (...
-
During the year 100,000 parts were handled 80,000 for regular stroller and 20,000 for jogging strollers 20,000 firmness tests were conducted 13,000 for regular stroller and 7,000 for jogging and...
-
Link from the text Web site to the site of the International Labour Organization, whose LABORSTA database reports consumer price indices for most of the worlds countries. For a recent year, identify...
-
For the function f(x) = x 2 , compute each average rate of change: (a) From 1 to 2 (b) From 1 to 1.5 (c) From 1 to 1.1 (d) From 1 to 1.01 (e) From 1 to 1.001 (f) Use a graphing utility to graph each...
-
Why does rebalancing work as a surrogate for misclassification costs? Use the following information for Exercises 2744. Suppose that our client is a retailer seeking to maximize revenue from a direct...
-
Warren Buffett, arguably the most famous investor in the United States, is the CEO of Berkshire Hathaway (BRK), a company that has enjoyed great success in terms of its stock price. Below are the...
-
a Dr The following is a summary of Benny Trading's Cash Book for the month of August 2021. Cheque No Cr 2021 2021 S Aug! Balance 4,800 Aug 4 Raymond 121 1.600 3 Rent 3,060 18 King Co. 122 4,268 14...
-
essay on the United Nations Human Rights Council's 2017 report on the financialization of housing (relevant links below). You may either agree or disagree with the report. Your grade should not...
-
The vaporization of ethanol at its normal boiling point, 351 K, has S = 110. J/K mol. Calculate E for the vaporization process at 1 atm and 351 K. CH5OH(1) CH5OH(g)
-
Tissue engineering involves the development of biological substitutes that restore or improve tissue function, Once manufactured, engineered organs can be implanted and grow within the patient,...
-
Cal Murphy is emigrating from Canada to take up residence in Jakarta, Indonesia. At the time of his departure, Cal will have the following Canadian assets: Cal has been impressed by the capital...
-
Why do you think diversity is important to organizations and what can a do to increase diversity in leadership? What is Servant Leadership? How can you apply this in your life? What is effective team...
-
How do you envision overcoming any potential resistance or skepticism from your colleagues in the vet tech field as you introduce these transformative strategies, and what steps do you think will be...
-
Managers encourage employees to do misleading activities such as speak falsehood and deceive customers which is clearly visible in the statement in the case " Sales are everything" wherein an...
-
Your Topic is "Why do you think there are so few people who succeed at both management and leadership? Is it reasonable to believe someone can be good at both?" Locate two to three articles about...
-
Explain the various benefits associated with professional networking. Also, expand on your answers how those would benefit you personally. PLEASE DO FAST AND CORRECT need correct answer
-
Solve the equation for the indicated variable. Use logarithms with the appropriate bases.
-
Evaluate the integral, if it exists. Jo y(y + 1) dy
-
Identify the indicated protons in the following molecules as unrelated, homotopic, enantiotopic, ordiastereotopic: (a) (b) Cysteine
-
The following 1H NMR absorptions were obtained on a spectrometer operating at 200MHz and are given in hertz downfield from the TMS standard. Convert the absorptions to units. (a) 436 Hz (b) 956 Hz...
-
The following 1H NMR absorptions were obtained on a spectrometer operating at 300MHz. Convert the chemical shifts from units to hertz downfield from TMS. (a) 2.1 (b) 3.45 (c) 6.30 (d) 7.70
-
Lou Barlow, a divisional manager for Sage Company, has an opportunity to manufacture and sell one of two new products for a five - year period. His annual pay raises are determined by his division s...
-
Consider a 5 year debt with a 15% coupon rate paid semi-annually, redeemable at Php1,000 par. The bond is selling at 90%. The flotation cost is Php50 per bind. The firm's tax bracket is 30%.
-
A project will generate annual cash flows of $237,600 for each of the next three years, and a cash flow of $274,800 during the fourth year. The initial cost of the project is $749,600. What is the...

Study smarter with the SolutionInn App


