The percent by mass of nitrogen for a compound is found to be 46.7%. Which of the
Question:
The percent by mass of nitrogen for a compound is found to be 46.7%. Which of the following could be this species?
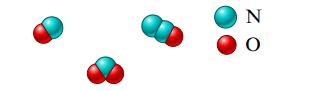
Transcribed Image Text:
N O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
The percent by mass of an element in a compound is ob...View the full answer

Answered By

Marvine Ekina
Marvine Ekina
Dedicated and experienced Academic Tutor with a proven track record for helping students to improve their academic performance. Adept at evaluating students and creating learning plans based on their strengths and weaknesses. Bringing forth a devotion to education and helping others to achieve their academic and life goals.
PERSONAL INFORMATION
Address: , ,
Nationality:
Driving License:
Hobbies: reading
SKILLS
????? Problem Solving Skills
????? Predictive Modeling
????? Customer Service Skills
????? Creative Problem Solving Skills
????? Strong Analytical Skills
????? Project Management Skills
????? Multitasking Skills
????? Leadership Skills
????? Curriculum Development
????? Excellent Communication Skills
????? SAT Prep
????? Knowledge of Educational Philosophies
????? Informal and Formal Assessments
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
The percent by mass of nitrogen for a compound is found to be 46.7%. Which of the following could be this species?
-
A mixture of NaBr and Na2 SO4 contains 29.96 percent Na by mass. Calculate the percent by mass of each compound in the mixture.
-
A sample of 0.6760 g of an unknown compound containing barium ions is dissolved in water and treated with an excess of Na2SO4. If the mass of the BaSO4 precipitate formed is 0.4105 g, what is the...
-
If a pure discount three-year bond sells for $782 and a pure discount four-year bond sells for $733, what is the expected 12-month interest rate in three years time? Both have a face value of $1000....
-
Carmen opens a retail store. Her sales during the first year are $600,000, of which $30,000 has not been collected at year-end. Her purchases are $400,000. She still owes $20,000 to her suppliers,...
-
Give an example where you made an attempt to personalize a message.
-
E3-2 General questions 1. Under GAAP, a parent company should exclude a subsidiary from consolidation if: a It measures income from the subsidiary under the equity method b The subsidiary is in a...
-
Lorance Corporation issued $800,000, 9%, 10-year bonds on January 1, 2014, for $750,150. This price resulted in an effective-interest rate of 10% on the bonds. Interest is payable semiannually on...
-
Nowak Carp prepar arterly and statements. The post closing trial balance at December 31, 2021, is presented below NOVAK CORP Post-Cloning Trial Balance December 31, 2021 Debit Credit $23.800 22.000...
-
Sarah recently sold her partnership interest for significantly more than her outside basis in the interest. Two separate appraisals were commissioned at the time of the sale to estimate the value of...
-
A compound that contains only carbon, hydrogen, and oxygen is 48.64% C and 8.16% H by mass. What is the empirical formula of this substance?
-
Complete the following table. Mass of Sample 4.24 g CH Moles of Sample 0.224 mol HO Molecules in Sample 2.71 X 1022 molecules CO Total Atoms in Sample 3.35 X 1022 total atoms in CH3OH sample
-
An experimenter is planning to do a random groups design experiment to study the effect of the rate of presenting stimuli on peoples ability to recognize the stimuli. The independent variable is the...
-
How do transnational organizations and agreements influence national sovereignty and political autonomy ?
-
How do individuals reconcile the tension between rational deliberation and emotional impulses when making consequential decisions amidst volatile environments, and to what extent does the phenomenon...
-
Watch the video clip below; https://www.youtube.com/watch?v=sE6Ox3ikCMU 1. Do you think that 'Rick and Morty' was a good choice? Justify your answer. 2. Do you think that this campaign will work for...
-
by the hypothesis that we want to do descriptive method, and quantative research in Tim hortons company, the question is A convincing closing statement, including that you'll develop your research...
-
Bottom of Form Why do you think ethics is important in healthcare management? What do you see as the biggest risks and temptations? How is your INTEGRITY a core principle in your professional ethical...
-
For a firm to be able to implement a strategy of price discrimination it must be able to prevent re-sale amongst its customers. What factors would tend to make it more difficult for a consumer to...
-
Find the image of x = k = const under w = 1/z. Use formulas similar to those in Example 1. y| y = 0 -21 -2 -1 -1, /1 12 T -1 -1 y= -2 x =0
-
Calculate the escape velocity (the minimum initial velocity that will take an object to infinity) from the surface of a planet of radius R. What is the value for? (a) The Earth, R = 6.37 X 106 m, g=...
-
The principal components of the atmosphere of the Earth are diatomic molecules, which can rotate as well as translate. Given that the translational kinetic energy density of the atmosphere is 0.15 J...
-
Enrico Fermi, the great Italian scientist, was a master at making good approximate calculations based on little or no actual data. Hence, such calculations are often called 'Fermi calculations'. Do a...
-
Due to the relationship of financial statements, the statement of stockholders' equity links the income statement to the balance sheet. True or False?
-
Troy Engines, Limited, manufactures a variety of engines for use in heavy equipment. The company has always produced all of the necessary parts for its engines, including all of the carburetors. An...
-
Trey is single and has no qualifying child. His adjusted gross income is $12,355. In order to claim the Earned Income Tax Credit, he must meet which of the following requirements? He cannot be the...

Study smarter with the SolutionInn App


