Complete the following table. Mass of Sample 4.24 g CH Moles of Sample 0.224 mol HO Molecules
Question:
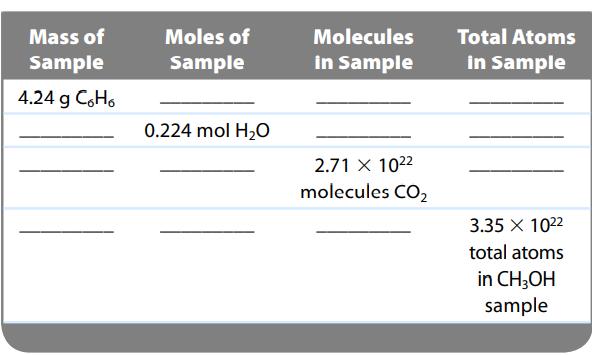
Complete the following table.
Transcribed Image Text:
Mass of Sample 4.24 g C₂H₂ Moles of Sample 0.224 mol H₂O Molecules in Sample 2.71 X 1022 molecules CO₂ Total Atoms in Sample 3.35 X 1022 total atoms in CH3OH sample
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
In order to complete the table we need to use some basic mole and stoichiometry calculations For the ...View the full answer

Answered By

Aketch Cindy Sunday
I am a certified tutor with over two years of experience tutoring . I have a passion for helping students learn and grow, and I firmly believe that every student has the potential to be successful. I have a wide range of experience working with students of all ages and abilities, and I am confident that I can help students succeed in school.
I have experience working with students who have a wide range of abilities. I have also worked with gifted and talented students, and I am familiar with a variety of enrichment and acceleration strategies.
I am a patient and supportive tutor who is dedicated to helping my students reach their full potential. Thank you for your time and consideration.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Complete the following table for a perfectly competitive firm, and indicate its profit-maximizingoutput. Total Marginal Marginal Quantity Price Revenue Revenue Total Cost Cos Total Profit $10 $30 35...
-
Complete the following table assuming that this project is in its fifthyear. PV of operating cash flows CBIT (1-T) Annual cash flow before taxesDiscount rate CCA rate Tax rate 24% 43% 36% 50% 15% 30%...
-
Complete the following table by entering either increases or decreases in columns (1) and (2), and either debit or credit in columns (3) and (4). (I) Debit (2) Credi (3)Increases (4) Decreases Assets...
-
Consider thedeadlock situation that could occur in the dining-philosophers problem when the philosophers obtain the chopsticks one at a time. Discuss how the four necessary conditions for deadlock...
-
For each of the following items, indicate whether the individual taxpayer must include any amount in gross income. a. Employees of Eastside Bookstore are given their birthdays off with pay. b....
-
Why can the Arabidopsis plant serve as a model for all flowering plants? Explain your answer.
-
5. Which of the following statements characterizes a consolidated entity? a It has an independent management board b It operates solely out of its headquarters c It does not exist d None of the above
-
Joan Ziemba owns a small variety store. The following transactions took place during March of the current year. Journalize the transactions in a general journal using the perpetual inventory method....
-
Finch Company makes three products in its factory: plastic cups, plastic tablecloths, and plastic bottles. The expected overhead costs for the next fiscal year include the following. Factory...
-
Find the absolute maximum and minimum of the function f(x, y) = r* + y* subject to the constraint a + y* = 4096. As usual, ignore unneeded answer blanks, and list points in lexicographic order....
-
The percent by mass of nitrogen for a compound is found to be 46.7%. Which of the following could be this species? N O
-
What amount (moles) is represented by each of these samples? a. 150.0 g Fe 2 O 3 b. 10.0 mg NO 2 c. 1.5 10 16 molecules of BF 3
-
Prove Theorem 2(b) and 2(c). Use the row-column rule. The (i, j)-entry in A(B+C) can be written as Data from in Theorem 2 " ai (bj + Cj) + ... + ain(bnj + Cnj) or aik (bkj + Ckj) k=1
-
Why do you think diversity is important to organizations and what can a do to increase diversity in leadership? What is Servant Leadership? How can you apply this in your life? What is effective team...
-
How do you envision overcoming any potential resistance or skepticism from your colleagues in the vet tech field as you introduce these transformative strategies, and what steps do you think will be...
-
Managers encourage employees to do misleading activities such as speak falsehood and deceive customers which is clearly visible in the statement in the case " Sales are everything" wherein an...
-
Your Topic is "Why do you think there are so few people who succeed at both management and leadership? Is it reasonable to believe someone can be good at both?" Locate two to three articles about...
-
Explain the various benefits associated with professional networking. Also, expand on your answers how those would benefit you personally. PLEASE DO FAST AND CORRECT need correct answer
-
Explain why the rise of e-commerce might increase the number of firms engaging in person-specific pricing.
-
All of the following assets can be depreciated, except: (a) A bulldozer (b) A copper mine (c) A surgical robot (d) A conveyor belt
-
In this problem, we examine a model for the transport of oxygen from air in the lungs to blood. First, show that, for the initial and boundary conditions c(x, t) = c(x, 0) = co (0 < x < ] and c(0, t)...
-
Consult literature sources and list the observed timescales during which the following processes occur radiative decay of excited electronic states, molecular rotational motion, molecular vibrational...
-
Describe the main features, including advantages and disadvantages, of the following experimental methods for determining the rate law of a reaction: the isolation method, the method of initial...
-
i need help in B and C Integrative Case 5-72 (Algo) Cost Estimation, CVP Analysis, and Decision Making (LO 5-4.5.9) Luke Corporation produces a variety of products, each within their own division....
-
Relate PSA (Public Securities Association) speed to the average life of a MBS. Describe the PSA measure and discuss which MBS would have the greater average life, one with a PSA of 100 or one with a...
-
Which of the following statement about swaps is least accurate? A. In a plain vanilla interest rate swap, the notional principal is swapped. B. The default problem [i.e. default risk] is the most...

Study smarter with the SolutionInn App


