Using the data in Figure 7-26 for an ironchromium- nickel alloy, determine the activation energy Qr and
Question:
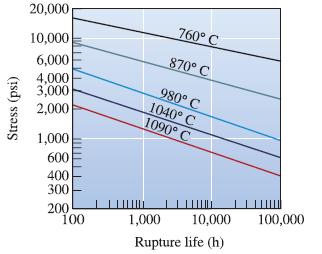
Using the data in Figure 7-26 for an ironchromium- nickel alloy, determine the activation energy Qr and the constant m for rupture in the temperature range 980 to 1090°C.
Transcribed Image Text:
Stress (psi) 20,000 10,000 6,000 4,000 3,000 2,000 1,000 600 400 300 200 100 760° C 870° C 980° C 1040° C 1090° C 1,000 10,000 Rupture life (h) 100,000
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
The activation energy Qr HcT where Hc RT ...View the full answer

Answered By

Willis Omondi
Hi, I'm Willis Omondi, a proficient and professional academic writer. I have been providing high-quality content that best suits my clients and completing their work within the deadline. All my work has been 100% plagiarism-free, according to research from my services, especially in arts subjects and many others
0.00
0 Reviews
10+ Question Solved
Related Book For 

The Science And Engineering Of Materials
ISBN: 9781305076761
7th Edition
Authors: Donald R. Askeland, Wendelin J. Wright
Question Posted:
Students also viewed these Engineering questions
-
7. Point P is located 1.7 m from fixed point charge q. The electric field E at point Pis 8.6 x 10-2 (1 point) N/C, directed away from the point charge. What is the magnitude of q? Let the...
-
Using the data in Solved Problem 10.1, prepare an activity chart like the one in the solved problem, but a second gas man also delivers 44 litres. In solved problem 10.1 Process Chart Summary O...
-
Using the data in BE21-4, compute equivalent units of production for materials and conversion costs, assuming materials are entered at the beginning of the process.
-
The management of a New York area investment firm wants to find out about the investment needs of its existing customers, for which it has an extensively detailed list, as a function of their...
-
Distinguish among a derivative action, dissent and oppression. Explain when it would be appropriate to use each of them.
-
What is the decision facing Airbus?
-
What are all the consequences of your decision, both negative and positive?(p. 208)
-
As immigrants continue to come to the United States from many different cultures and religions, differences will cause some challenges and problems. One area where this has occurred is with Islamic...
-
Question # 21 - The following information is to be used for questions 21 through 25 Starship Corp. acquired 100% of Jefferson Inc. on April 1, 2018 for $1,300,000. Payment was in the form of $600,000...
-
At the beginning of 2021, Thompson Service, Inc., showed the following amounts in the stockholders equity section of its balance sheet. The transactions relating to stockholders equity during the...
-
Approximate the temperature at which creep deformation becomes an important consideration for each of the following metals: tin, molybdenum, iron, gold, zinc and chromium.
-
What is the difference between failure of a material by creep and that by stress rupture?
-
Define product differentiation, describe 11 bases of product differentiation, and how these bases of product differentiation can be grouped into three categories.
-
What is the difference between corporate and clinical? How do they differ? Can they both have the same outcome? Include a reference list that supports your stance of no fewer than 3 scholarly...
-
How do we attain the desire for the freedom to purse one's passions, the desire for economic security and well-being, the desire for hope and progress in one's life utilizing higher-order thinking
-
Instructions FNCE 625 - Investment Analysis and Management Group Project - Case Study Guideline Introduction: In this group assignment, each team will collaboratively make a comprehensive report and...
-
21) The EOQ model is solved using calculus but the key intuition is that relevant total costs are minimized when relevant ordering costs equal relevant carrying costs. 22) Safety stock is used as a...
-
In the long-term, what do you recommend as overall policy in order to reduce or avoid the kinds of PPE shortages that occurred during the different waves of the COVID virus? In simple terms, how...
-
Show that the graph of the equation x 1/2 + y 1/2 = a 1/2 is part of the graph of a parabola.
-
MgO prevents premature evaporation of Al in a furnace by maintaining the aluminum as Al2O3. Another type of matrix modifier prevents loss of signal from the atom X that readily forms the molecular...
-
Specifications for lactated Ringers solution, which is used for intravenous (IV) injections, are as follows to reach 100. mL of solution: 285315 mg Na + 14.117.3 mg K + 4.96.0 mg Ca 2+ 368408 mg Cl -...
-
You have a solution of two volatile liquids, A and B (assume ideal behavior). Pure liquid A has a vapor pressure of 350.0 torr and pure liquid B has a vapor pressure of 100.0 torr at the temperature...
-
A solid mixture contains MgCl 2 and NaCl. When 0.5000 g of this solid is dissolved in enough water to form 1.000 L of solution, the osmotic pressure at 25.0 C is observed to be 0.3950 atm. What is...
-
An 8%, 30-year semi-annual corporate bond was recently being priced to yield 10%. The Macaulay duration for this bond is 10.2 years. What is the bonds modified duration? How much will the price of...
-
Question 7 of 7 0/14 W PIERDERY Current Attempt in Progress Your answer is incorrect Buffalo Corporation adopted the dollar value LIFO retail inventory method on January 1, 2019. At that time the...
-
Cost of debt with fees . Kenny Enterprises will issue a bond with a par value of $1,000, a maturity of twenty years, and a coupon rate of 9.9% with semiannual payments, and will use an investment...

Study smarter with the SolutionInn App


