What are the names of the compounds that correspond to the formulas given in the following table?
Question:
What are the names of the compounds that correspond to the formulas given in the following table?
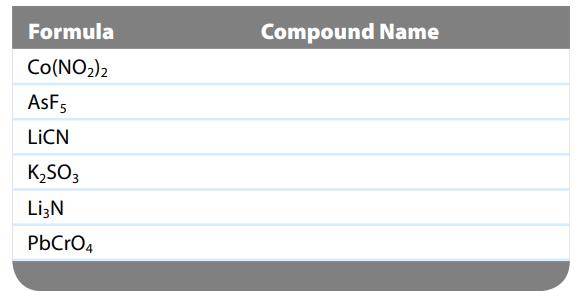
Transcribed Image Text:
Formula Co(NO₂)2 AsF5 LICN K₂SO3 Li3N PbCrO4 Compound Name
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
ANSWER CoNO22 Cobalt II Nitrite AsF5 Arsenic Pentafluoride LICN Lithium Cyanide KSO...View the full answer

Answered By

Churchil Mino
I have been a tutor for 2 years and have experience working with students of all ages and abilities. I am comfortable working with students one-on-one or in small groups, and am able to adapt my teaching style to meet the needs of each individual. I am patient and supportive, and my goal is to help my students succeed.
I have a strong background in math and science, and have tutored students in these subjects at all levels, from elementary school to college. I have also helped students prepare for standardized tests such as the SAT and ACT. In addition to academic tutoring, I have also worked as a swim coach and a camp counselor, and have experience working with children with special needs.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
What are the formulas of the compounds that correspond to the names given in the following table? Compound Name Carbon tetrabromide Cobalt(II) phosphate Magnesium chloride Nickel(II) acetate Calcium...
-
Explain the Component Diagram of Bug Tracker in detail.
-
What are the names of three national labor unions that you are aware of and what are the general work activities of their members?
-
Prove that opposite sides of a quadrilateral circumscribing a circle subtend supplementary angles at the centre of the circle.
-
Sally is an attorney who computes her taxable income using the cash method of accounting. Sage Corporation owned 40% by Sallys brother, 40% by her cousin, and 20% by her grandmother, uses the accrual...
-
Will different methods of evaluating a project typically yield the same conclusions about whether to fund its development? Why or why not?
-
Facial features of successful financial analysts. Refer to the Journal of Accounting Research (September 2019) study of the facial features of successful financial analysts, Exercise 12.70 (p. 759)....
-
Does the reaction p + 7/3Li 4/2He + required energy, or does it release energy? How much energy?
-
to the industry average? A. higher asset fumowir B. conribtent movement between sabos. merchandive inventory, and recelvables on the francial statements C. higher times - intorest earned D. fower...
-
If you drop a piece of buttered toast on the floor, is it just as likely to land buttered side up as buttered side down? It sure seems like mine always lands buttered side down! Suppose that 7 of the...
-
Which of the following statements is(are) true? For the false statements, correct them. a. All particles in the nucleus of an atom are charged. b. The atom is best described as a uniform sphere of...
-
What are the symbols for the following nonmetal elements that are most often present in compounds studied in organic chemistry: carbon, hydrogen, oxygen, nitrogen, phosphorus, sulfur? Predict a...
-
Repeat the previous exercise, but with a class Number where T can be any numeric type. Try adding % to Number and see what happens when you try to use % for Number and Number. Data from Previous...
-
In 2020 the global distribution of sales in the industrial gas industry was as follows: i What is Air Liquides position on a GCI/GRI mapping? Global industrial gas industry 82 billion The 2020 global...
-
The General Social Survey polled a sample of 1048 adults in the year 2010, asking them how many hours per week they spent on the Internet. The sample mean was 9.79 with a standard deviation of 13.41....
-
An article in the Archives of Internal Medicine reported that in a sample of 244 men, 73 had elevated total cholesterol levels (more than 200 milligrams per deciliter). In a sample of 232 women, 44...
-
Explain how search can be used to solve constraint satisfaction problems, such as the eight-queens problem. What difficulties arise when such problems become extremely large (e.g., the...
-
Casse (1981) developed an exercise to encourage his students to develop their empathic skills. He asked them to listen to a recording of a dialogue between John Miller (a US project manager in...
-
1. Would it matter if it were easy to forge a 10 note but cost 15 to do so? 2. How well do each of the following meet the seven requirements listed above: grain, strawberries, strawberry jam, gold,...
-
Use nodal analysis to determine voltages v1, v2, and v3 in the circuit Fig. 3.76. Figure 3.76 4 S 3i, 2 A 4A
-
The standard enthalpy of a certain reaction is approximately constant at + 125 k] mol-l from 800 K up to 1500 K. The standard reaction Gibbs energy is +22 kJ mol-1 at 1120 K. Estimate the temperature...
-
The equilibrium constant of a reaction is found to fit the expression In K =A + BIT+ CIT3 between 400 K and 500 K with A = -2.04, B =-1176 K, and C = 2.1 X 107 K3 Calculate the standard reaction...
-
The equilibrium pressure of H, over solid uranium and uranium hydride, UH3' at 500 K is 139 Pa. Calculate the standard Gibbs energy of formation ofUH3 (s) at 500 K.
-
All else constant, if the yield to maturity of a bond increases, the the value of the bond __________. a. increases b. decreases c. remains the same d. not enough information To answer enter a, b, c,...
-
Martha s Vineyard Marine Supply is a wholesaler for a large variety of boating and fishing equipment. The company s controller, Mathew Knight, has recently completed a cost study of the firm s...
-
1. Compute the productivity profiles for each year. If required, round your answers to two decimal places. 2a. Did productivity improve? 2b. Explain why or why not

Study smarter with the SolutionInn App


