Write the balanced formula, complete ionic, and net ionic equations for each of the following acidbase reactions.
Question:
Write the balanced formula, complete ionic, and net ionic equations for each of the following acid–base reactions.
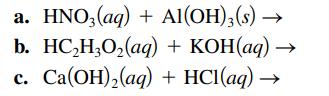
Transcribed Image Text:
a. HNO3(aq) + Al(OH)3(s) → b. HC₂H₂O₂(aq) + KOH(aq) → C. Ca(OH)₂(aq) + HCl(aq) →
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
a HNO3aq AlOH3s AlNO33aq H2Ol Complete ionic equation H...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Write the balanced molecular and net ionic equations for each of the following neutralization reactions: (a) Aqueous acetic acid is neutralized by aqueous barium hydroxide. (b) Solid chromium(III)...
-
For the reactions in Exercise 52, write the balanced formula equation, complete ionic equation, and net ionic equation. If no precipitate forms, write No reaction. DATA IN Exercise 52, When the...
-
Write the balanced formula, complete ionic, and net ionic equations for each of the following acidbase reactions. a. HCIO4 (aq) + Mg(OH)(s) b. HCN(aq) + NaOH(aq) C. HCl(aq) + NaOH(aq)
-
Consider sending a large file from a host to another over a TCP connection that has no loss. a. Suppose TCP uses AIMD for its congestion control without slow start. Assuming cwnd increases by I MSS...
-
Kim leased an office building to USA Corporation under a ten-year lease specifying that at the end of the lease USA had to return the building to its original condition if any modifications were...
-
Instead of regressing LDIVIDENDS on LCP in level form, suppose you regress the first difference of LDIVIDENDS on the first difference of LCP. Would you include the intercept in this regression? Why...
-
A company is engaged in producing a standard mix using 60 kg of Material X and 40 kg of Material Y. The standard loss of production is 30%. The standard price of X is Rs. 5 per kg and of Y is Rs. 10...
-
In the early 1970s a widely publicized list of the "Nifty Fifty" stocks was drawn up. This list, which included Avon Products, Polaroid, Coca-Cola, McDonald's, Walt Disney, American Express, and...
-
Town Company had the following partially completed payroll register Click the icon to view the partial payroll register) (Click the icon to view payroll tax rate information) Read the requirements...
-
Using the What Do Operations Managers Do? box in the chapter, what key activities most directly relate to the case situation?
-
You are given a 1.50-g mixture of sodium nitrate and sodium chloride. You dissolve this mixture into 100 mL of water and then add an excess of 0.500 M silver nitrate solution. You produce a white...
-
A 1.42-g sample of a pure compound, with formula M 2 SO 4 , was dissolved in water and treated with an excess of aqueous calcium chloride, resulting in the precipitation of all the sulfate ions as...
-
Explain the items reported on a statement of retained earnings.
-
Write out the form of the partial fraction decomposition of the function (see example). Do not determine the numerical values of the coefficients. x3 (a) x + 7x+6 9x+1 (b) (x + 1)3(x + 2) Submit...
-
You desire to make an 80% by weight vinyl acetate to 20% by weight styrene copolymer via free radical, emulsion polymerization. The r 1 and r 2 values for these monomers are 0.01 and 55,...
-
Q1)In a wheel and axle machine the diameters of the wheel and the axle are 450mm and 60mm respectively.The efficiency is 97%(0.97 per unit).When a body having a mass of 40kg is being lifted.Determine...
-
Smith & Chief Ltd. of Sydney, Australia, is a merchandising firm that is the sole distributor of a product that is increasing in popularity among Australian consumers. The company's income statements...
-
C. In lab, you measure the x & y components of a possible incompressible flow field as u = 2cxy; and where cand a are constants. v = c(a + x - y) 5. (04 pts) Short answer, what is necessary for the...
-
Assume that it is agreed by everyone that it is morally wrong to treat labour as a mere factor of production, with no rights over the goods produced. Does this make the neoclassical theory wrong?
-
As economic conditions change, how do banks adjust their asset portfolio?
-
When an electron occupies a 2s orbital on an N atom it has a hyper fine interaction of 55.2 mT with the nucleus. The spectrum of N02 shows an isotropic hyper fine interaction of 5.7 mT. For what...
-
When an electron occupies a 2s orbital on an N atom it has a hyperfine interaction of 55.2 mT with the nucleus. The spectrum ofN02 shows an isotropic hyperfine interaction of 5.7 mT. For what...
-
The z-component of the magnetic field at a distance R from a magnetic moment parallel to the z-axis is given by eqn 15.28. In a solid, a proton at a distance R from another can experience such a...
-
Eye Deal Optometry leased vision - testing equipment from Insight Machines on January 1 , 2 0 2 4 . Insight Machines manufactured the equipment at a cost of $ 2 0 0 , 0 0 0 and lists a cash selling...
-
help! ee all photos + Add to o e D C N X Edit & Create Share Table of Contents No sales to an individual customer accounted for more than 10% of revenue during any of the last three fiscal years. Net...
-
Business law A person may have the liability of a partner even though no partnership exists True False

Study smarter with the SolutionInn App


