Write the balanced formula, complete ionic, and net ionic equations for each of the following acidbase reactions.
Question:
Write the balanced formula, complete ionic, and net ionic equations for each of the following acid–base reactions.
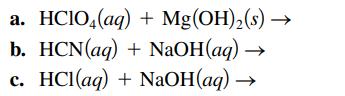
Transcribed Image Text:
a. HCIO4 (aq) + Mg(OH)₂(s) → b. HCN(aq) + NaOH(aq) C. HCl(aq) + NaOH(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 69% (13 reviews)
a HClO4aq MgOH2s MgClO4aq H2Ol Complete ionic equati...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Write the balanced molecular and net ionic equations for each of the following neutralization reactions: (a) Aqueous acetic acid is neutralized by aqueous barium hydroxide. (b) Solid chromium(III)...
-
For the reactions in Exercise 52, write the balanced formula equation, complete ionic equation, and net ionic equation. If no precipitate forms, write No reaction. DATA IN Exercise 52, When the...
-
Write the balanced formula, complete ionic, and net ionic equations for each of the following acidbase reactions. a. HNO3(aq) + Al(OH)3(s) b. HCHO(aq) + KOH(aq) C. Ca(OH)(aq) + HCl(aq)
-
If you were a hedge fund manager, which style would you employ and why? Describe this style and how it works. Why would you utilize it and under what macroeconomic conditions would this style work...
-
Ann is a graduate economics student at State University. State University awarded her a $1,000 scholarship. In addition, Ann works as a half-time teaching assistant in the Economics Department at...
-
From the U.K. private sector housing starts (X) for the period 1948 to 1984, Terence Mills obtained the following regression results: The 5 percent critical Ï value is 2.95 and the 10 percent...
-
From the following information, calculate labour variances: Actual wage paid Rs. 6000; Standard hours 3,200; Standard hourly rate Rs. 1.50; Actual hours paid 3,000 hrs Idle Time 100 hours...
-
Many companies use budgets for three purposes. First, they use them to plan how to deploy resources to best serve customers. Second, they use them to establish challenging goals, or stretch targets,...
-
The last dividend paid by Coppard Inc. was $1.75. The dividend growth rate is expected to be constant at 15% for 3 years, after which dividends are expected to grow at a rate of 6% forever. If the...
-
Hector Fuego had just been hired by the board of directors to become the new CEO of You Build, Inc. You Build is a 50-year-old building supply company that operates in the southwestern United States....
-
You are given a 1.50-g mixture of sodium nitrate and sodium chloride. You dissolve this mixture into 100 mL of water and then add an excess of 0.500 M silver nitrate solution. You produce a white...
-
A 1.42-g sample of a pure compound, with formula M 2 SO 4 , was dissolved in water and treated with an excess of aqueous calcium chloride, resulting in the precipitation of all the sulfate ions as...
-
Kerry owns the concession to run a souvenir shop at a tourist location near London. She sells souvenir tea towels, cards, biscuits, and various toys and gifts. Kerry's sales for the year to 30...
-
Year 5% 6% 4 3.546 3.465 5 7% 3.387 3.312 4.329 4.212 4.100 8% 3.993 5.076 4.917 4.767 4.623 Present Value of an Annuity of $1 at Compound Interest 9% 10% 11% 12% 13% 14% 15% 3.240 3.170 3.102 3.037...
-
2. Determine the overturning stability of the cantilever retaining wall shown. The equivalent fluid density is 5.5 kN/m, soil density is 18 kN/m, and the concrete weighs 23.5 kN/m. (5 pts) 2 m 2 m 2...
-
A. For a certain two-dimensional, incompressible flow field the velocity component in the y direction is given by v = 3xy + xy 1. (05 pts) Short answer, what is the condition for this flow field to...
-
Cho0se a hazardous material to cr3ate a pr3sentation on (i.e. sulfuric acid, explosives, used needles, there are many types of hazardous materials) Cr3ate a presentation (P0werPoint, Open0ffice...
-
If det [a b] = c d 2 -2 0 a. det c+1 -1 2a d-2 2 2b -2 calculate:
-
1. Which of the following is more likely to be consistent with the aim of maximising profits: pricing on the basis of (a) cost per unit plus a variable percentage mark-up; (b) cost per unit plus a...
-
In what ways does a well-designed enterprise search software vary from popular search engines (e.g., Bing, DuckDuckGo, and Google)?
-
The shape of a spectral line, J(w), is related to the free induction decay signal G(t) by where a is a constant and 'Re' means take the real part of what follows. Calculate the lineshape...
-
EPR spectra are commonly discussed in terms of the parameters that occur in the spin-Hamiltonian, a Hamiltonian operator that incorporates various effects involving spatial operators (like the...
-
When interacting with a large biopolymer or even larger organelle, a small molecule might not rotate freely in all directions and the dipolar interaction might not average to zero. Suppose a molecule...
-
The tolal landed coet with the order gaantly sire of 6,000 unts is 4 (Enter your response roundod to the nearest dolar)
-
Boyne Inc. had beginning inventory of $12,000 at cost and $20,000 at retail. Net purchases were $120,000 at cost and $170,000 at retail. Net markups were $10,000, net markdowns were $7,000, and sales...
-
Apple inc. CONDENSED CONSOLIDATED BALANCE SHEETS (Unaudited) (In milions, except number of shares which are reflected in thousands and par value) LABILITES AND SHAREHOLDERS' EQUITY: Current...

Study smarter with the SolutionInn App


