You have liquid in each graduated cylinder shown: You then add both samples to a beaker. How
Question:
You have liquid in each graduated cylinder shown:
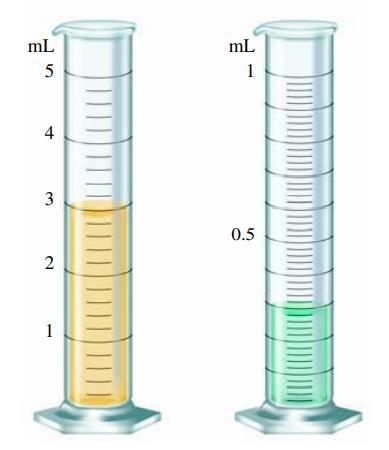
You then add both samples to a beaker. How would you write the number describing the total volume? What limits the precision of this number?
Transcribed Image Text:
mL 5 4 3 2 1 www mL 1 0.5
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a I would write by addin...View the full answer

Answered By

Willis Omondi
Hi, I'm Willis Omondi, a proficient and professional academic writer. I have been providing high-quality content that best suits my clients and completing their work within the deadline. All my work has been 100% plagiarism-free, according to research from my services, especially in arts subjects and many others
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
You have a graduated cylinder that contains a liquid (see photograph). Write the volume of the liquid, in milliliters, using the proper number of significant figures. 21
-
Write an essay describing how business decision support systems have evolved over the past several decades as computer and data capabilities have grown. In order to built your understanding read "A...
-
. How would you write the set Q({,{,{}}}) in list notation?
-
Find the minimum value of the function: f(x) = x^4 - 6x^2 + 9 on the interval [-2,2].
-
Dean makes a pledge of $30,000 to a local college. The college is willing to accept either cash or marketable securities in fulfillment of the pledge. Dean owns stock in Ajax Corporation worth...
-
The box beam is subjected to a moment of M = 15 kip ft. Determine the maximum bending stress in the beam and the orientation of the neutral axis. y 4 in. 4 in. 6 in. ID -6 in.-
-
User experience professionals salary survey. The User Experience Professionals Association (UXPA) supports people who research, design, and evaluate the user experience of products and services. The...
-
At the beginning of 2014, Florida Rock Industries had 25,000 shares of common stock issued and outstanding and 500 $1,000, 6% bonds, each convertible into 10 shares of common stock . During 2014,...
-
Question 3 (6 points) Grower Company produces two products from a common input. Current data is as follows: Product X Product Z Number of units produced 10,000 5,000 Joint processing costs $30,000...
-
Branch Manufacturing Corporation owns 80 percent of the common shares of Short Retail Stores. The companies balance sheets as of December 31, 20X4, were as follows: Short Retail's 8 percent preferred...
-
Is the density of a gaseous substance larger or smaller than the density of a liquid or a solid at the same temperature? Why?
-
Which of the following are exact numbers? a. There are 100 cm in 1 m. b. One meter equals 1.094 yards. c. We can use the equation to convert from Celsius to Fahrenheit temperature. Are the numbers...
-
Distinguish between public debt and a deficit.
-
Most businesses have been impacted negatively in 2020 by the outbreak of Corona virus leading to the disease Covid 19. Many countries went in lock down where by economic activities nearly came to a...
-
The unadjusted trial balance has been entered on a 10-column end-of-period spreadsheet work sheet) for you. Complete the spreadsheet using the following adjustment data a Physcial inventory count on...
-
A) What should be the price of the call option? B) Assume that the call option on Apple with strike price $90 and maturity in one year is currently trading at $17. You immediately tell your broker...
-
White Company has two departments, Cutting and Finishing. The company uses job-order costing and computes a predetermined overhead rate in each department. The Cutting Department bases its rate on...
-
Can someone please help me figure out how to find the qualified business income for this problem? Maria and Javier are the equal partners in MarJa, a partnership that is a qualifying trade or...
-
1. Under what circumstances is a country likely to have an elastic demand for (a) its exports; (b) its imports? 2. Over which time period is the MarshallLerner condition less likely to be met: the...
-
Which of the following raises the credibility of areport? Which of the following raises the credibility of a report? Multiple Choice avoiding predictions avoiding the use of cause-effect statements...
-
Set up a thermodynamic cycle for determining the enthalpy of hydration of Ca2+ ions using the following data: enthalpy of sublimation of Ca (s), + 178.2 k] mol-1, first and second ionization...
-
A vapour at 22 atm and 5C was allowed to expand adiabatically to a final pressure of 1:00 atm; the temperature fell by 10 K. Calculate the Joule- Thomson coefficient, u, at 5C, assuming it remains...
-
Repeat Exercise 2.30(a) for argon, from an initial volume of 1.00 dm3 to 22.1 dm3 at 298 K.
-
why would an auditor want to complete dual-purpose tests? what procedure can be put into place to help prevent fraud? List 4 procedures.
-
Based on the following information, calculate sustainable growth rate for Groot, Inc.: Profit margin= 7.1% Total asset turnover = 1.90 Total debt ratio = .45 Payout ratio = 20% What is the ROA here?
-
Consider the following: a call option on a stock has strike price $100, premium of $5 and the current price of the underlying stock is $100. If you buy the call option today, what is your holding...

Study smarter with the SolutionInn App


