Question: A 3-ft3 adiabatic rigid container is divided into two equal volumes by a thin membrane, as shown in Fig. P473E. Initially, one of these chambers
A 3-ft3 adiabatic rigid container is divided into two equal volumes by a thin membrane, as shown in Fig. P4–73E. Initially, one of these chambers is filled with air at 100 psia and 100°F while the other chamber is evacuated. Determine the internal energy change of the air when the membrane is ruptured. Also determine the final air pressure in the container.
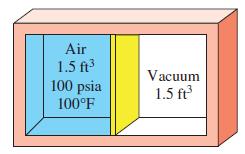
Air 1.5 ft 100 psia Vacuum 1.5 ft 100F
Step by Step Solution
3.36 Rating (168 Votes )
There are 3 Steps involved in it
Assumptions 1 Air is an ideal gas since it is at a high temperature and low ... View full answer

Get step-by-step solutions from verified subject matter experts


