Question: Determine the total heat transfer for the reversible process 1-2 shown in Fig. P759. 500 100 0.2 1.0 S, kJ/K T, C
Determine the total heat transfer for the reversible process 1-2 shown in Fig. P7–59.
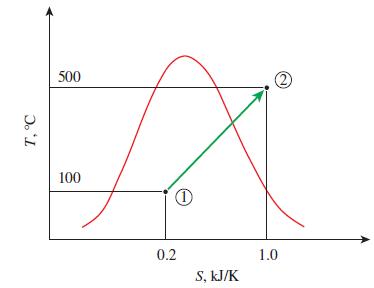
500 100 0.2 1.0 S, kJ/K T, C
Step by Step Solution
★★★★★
3.37 Rating (172 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

The total heat transfer fot the process 12 shown ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


