In Prob. 719, assume that the heat is transferred from the cold reservoir to the hot reservoir
Question:
In Prob. 7–19, assume that the heat is transferred from the cold reservoir to the hot reservoir contrary to the Clausius statement of the second law. Prove that this violates the increase of entropy principle—as it must according to Clausius.
Data From Q#19:
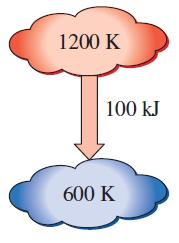
Heat in the amount of 100 kJ is transferred directly from a hot reservoir at 1200 K to a cold reservoir at 600 K. Calculate the entropy change of the two reservoirs and determine if the increase of entropy principle is satisfied.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Thermodynamics An Engineering Approach
ISBN: 9781259822674
9th Edition
Authors: Yunus Cengel, Michael Boles, Mehmet Kanoglu
Question Posted:





