Reconsider Prob. 1443. Determine the adiabatic saturation temperature of the humid air. Data From Prob. 1443. Atmospheric
Question:
Reconsider Prob. 14–43. Determine the adiabatic saturation temperature of the humid air.
Data From Prob. 14–43.
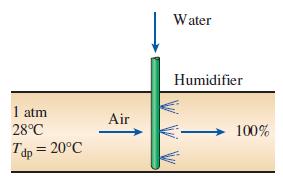
Atmospheric air at a pressure of 1 atm and dry-bulb temperature of 28°C has a dew-point temperature of 20°C. Using the psychrometric chart.
Transcribed Image Text:
Water Humidifier 1 atm 28°C Air 100% Tdp = 20°C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
The pressure temperature and wetbulb temperature of air are specified The adiabatic sa...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Thermodynamics An Engineering Approach
ISBN: 9781259822674
9th Edition
Authors: Yunus Cengel, Michael Boles, Mehmet Kanoglu
Question Posted:
Students also viewed these Engineering questions
-
Determine the adiabatic saturation temperature of the humid air in Prob. 14-41. Prob. 14-41. Atmospheric air at a pressure of 1 atm and drybulb temperature of 28oC has a wet-bulb temperature of 20oC....
-
Determine the adiabatic saturation temperature of the humid air in Prob. 14-43E. Prob. 14-43E Atmospheric air at a pressure of 1 atm and drybulb temperature of 90oF has a dew-point temperature of...
-
Reconsider Prob. 1439E. Determine the adiabatic saturation temperature of the humid air. Data From Prob. 1439E: Atmospheric air at a pressure of 1 atm and dry-bulb temperature of 90F has a wet-bulb...
-
Presented below is information related to LeBron James Manufacturing Corporation. Instructions (a) Compute the rate of depreciation per year to be applied to the plant assets under the composite...
-
The number 22/7 is similar to TT in the sense that they both require an infinite number of decimal places. However, 22/7 is a rational number because it can be expressed as the ratio of two integers,...
-
Do marketing employees use appropriate marketing planning and controlling tools and techniques?
-
Bonds with the same term to maturity but with smaller coupons have __________ price volatility when interest rates change.
-
Tomkung Corporations income statements are presented in the following spreadsheet. Required Construct a spreadsheet to conduct horizontal analysis of the income statements for 2015 and2014. 2015 2014...
-
The comparative statements of Oriole Company are presented here. ORIOLE COMPANY Income Statements For the Years Ended December 31 2020 $1,895,640 1,063,640 2019 $1,755,600 1,011, 100 744,500 484,100...
-
Spring Garden Preschool operates a not-for-profit morning preschool. Each family pays a nonrefundable registration fee of $120 per child per school year. Monthly tuition for the eight-month school...
-
What does a modern air-conditioning system do besides heating or cooling the air?
-
Atmospheric air at a pressure of 1 atm and dry-bulb temperature of 90F has a wet-bulb temperature of 85F. Using the psychrometric chart, determine (a) The relative humidity (b) The humidity ratio (c)...
-
Psychologists interested in learning theory study learning curves. A learning curve is the graph of a function, the performance of someone learning a skill as a function of the training time t. The...
-
Small town Diners has a policy of treating dividends as a passive residual. It forecasts that net earnings after taxes in the coming year will be $500,000. The firm has earned the same $500,000 for...
-
Part 1-Chi-Square Goodness-of-Fit Tests A health psychologist was interested in women's workout preferences. Of the 56 participants surveyed, 22 preferred running, 8 preferred swimming, 15 preferred...
-
The Campbell Company is considering adding a robotic paint sprayer to its production line. The sprayer's base price is $1,070,000, and it would cost another $21,000 to install it. The machine falls...
-
Problem 1. (10 points) Consider the space X = R22 and the map L XX defined as traceX -traceX L:X X = X 0 0 1. Show that L is a linear map; 2. Find the matrix representation M = mat L in the canonical...
-
Suppose that the exchange rate is 1.25 = 1.00. Options (calls and puts) are available on the Philadelphia exchangein units of10,000 with strike prices of $1.60/1.00. Options (calls and puts) are...
-
Repeat Prob. 51 with the following principal stresses obtained from Eq. (313) : (a) A = 100 MPa, B = 100 MPa (b) A = 100 MPa, B = -100 MPa (c) A = 100 MPa, B = 50 MPa (d) A = 100 MPa, B = -50...
-
Find the image of x = k = const under w = 1/z. Use formulas similar to those in Example 1. y| y = 0 -21 -2 -1 -1, /1 12 T -1 -1 y= -2 x =0
-
The volumetric analysis of a mixture of gases is 30 percent oxygen, 40 percent nitrogen, 10 percent carbon dioxide, and 20 percent methane. This mixture flows through a 1.6-cm-diameter pipe at 8000...
-
A rigid tank contains 1 lbmol of argon gas at 400 R and 750 psia. A valve is now opened, and 3 lbmol of N2 gas is allowed to enter the tank at 340 R and 1200 psia. The final mixture temperature is...
-
The volumetric analysis of mixture of gases is 30 percent oxygen, 40 percent nitrogen, 10 percent carbon dioxide, and 20 percent methane. This mixture is heated from 20oC to 200oC while flowing...
-
How to solve general ledger cash balance chapter 9 assignment 5
-
On 31 July 2018, Sipho bought 1 000 ordinary shares in ABC Ltd at a cost of R2 750. On 31 December 2018 the company made a 1 for 10 bonus issue. On 31 March 2019, Sipho sold 300 shares for R800. What...
-
If you purchase a $1000 par value bond for $1065 that has a 6 3/8% coupon rate and 15 years until maturity, what will be your annual return? 5.5% 5.9% 5.7% 6.1%

Study smarter with the SolutionInn App


