Consider an ideal vapor-compression cycle using R-134a and operating between pressures of 0.28 and 1.75 MPa. Determine
Question:
Consider an ideal vapor-compression cycle using R-134a and operating between pressures of 0.28 and 1.75 MPa. Determine the coefficient of performance for the cycle for application
(a) In a refrigerator
(b) In a heat pump.
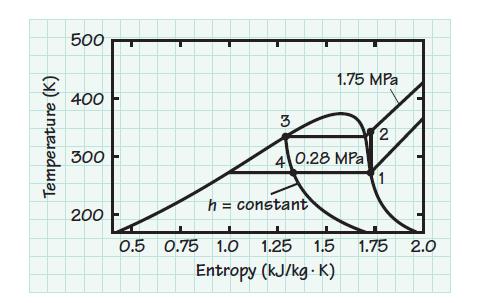
Transcribed Image Text:
Temperature (K) 500 400 300 200 0.5 3 0.75 1.0 h = constant 1.75 MPa 4 0.28 MPa 1.25 1.5 Entropy (kJ/kg. K) 2 1 1.75 2.0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
To determine the coefficient of performance COP for the ideal vaporcompression cycle using R134a we ...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
Determine the heat pump coefficient of performance for an ideal vapor compression refrigeration cycle operating between 0.653 and 1.342 MPa. The working fluid is R-22. Compare your result with that...
-
A heat pump that operates on the ideal vapor compression cycle with refrigerant-134a is used to heat a house and maintain it at 75F by using underground water at 50F as the heat source. The house is...
-
A refrigerator uses R-134a as the working fluid and operates on an ideal vapor compression refrigeration cycle between 0.15 MPa and 1 MPa. A temperature difference of 5C is maintained for effective...
-
- The first stream is $450 per year for 9 years and begins 5 years from today - The second stream begins 7 years from today with the first cash flow being $400, and with each successive cash flow...
-
The Alaska Airlines balance sheet dated December 31, 2011, included the following ($ in millions): Property and equipment Aircraft and other flight equipment ........$4,041.8 Other property and...
-
If Y is distributed N(-3,4), Pr (Y> -5) = (Round your response to four decimal places.)
-
Reconstructing underlying events from ending inventory amounts. (Adapted from CPA examination.) Burch Corporation began a merchandising business on January 1. Year 1. It acquired merchandise costing...
-
The term churn is very important to managers in the cellular phone business. Churning occurs when a customer stops using one companys service and switches to another companys service. Obviously,...
-
Cherokee Inc. is a merchandiser that provided the following information: Amount 11,000 Number of units sold Selling price per unit Variable selling expense per unit Variable administrative expense...
-
The Edwards Lake Community Hospital balance sheet as of December 31, 2016, follows. Required a. Record in general journal form the effect of the following transactions during the fiscal year ended...
-
Repeat Problem 9.117 using EES. Modify your EES program to find the coefficient of performance between pressures of 0.28 and 1.5 MPa. Plot Ts diagrams for the original cycle conditions and for the...
-
Heat leaks from the air in a kitchen through the walls of a refrigerator into the refrigerated cold space at a rate of 1.43 kW. Determine the electrical power required to maintain a steady...
-
Find each of these values? a) (133 mod 23 + 261 mod 23) mod 23 b) (457 mod 23 182 mod 23) mod 23
-
You have recently taken over daycare center that was under substandard leadership. Currently, the staff is unmotivated, negative, and often absent from work. You notice that there is minimal...
-
Choose an organization from the industry of your choice to discuss, illustrate, and reflect deliberately on the following: Why is it important to distinguish between "group" and "team "? What kinds...
-
The focus of data governance programs, in some capacity, is enterprise-wide data quality standards and processes. If you were a manager focusing on master data: Would you likely meet enterprise-level...
-
1) Identify and explain each component of the ANOVA model. 2) How is the F ratio obtained? 3) What role does the F ratio play?
-
Make a BCG matrix table and place the following products from Apple: iPhone, iPad, iMac, iPod, Apple TV, Apple Watch, AirPod, and HomePod. Briefly describe why you have placed the products in the...
-
Identify the mechanism expected to operate when 2-bromo-2-methylpentane is treated with each of the following reagents: a) EtOH b) t-BuOK c) NaI d) NaOEt e) NaOH
-
Why do CPA firms sometimes use a combination of positive and negative confirmations on the same audit?
-
A spring-loaded pistoncylinder device contains 5 kg of helium as the system, as shown in Fig. P477. This system is heated from 100 kPa and 20C to 800 kPa and 160C. Determine the heat transferred to...
-
A balloon initially contains 40 m 3 of helium gas at atmospheric conditions of 100 kPa and 17C. The balloon is connected by a valve to a large reservoir that supplies helium gas at 125 kPa and 25C....
-
Water is boiled at 100C electrically by a 3-kW resistance wire. Determine the rate of evaporation of water. Steam Water 100C
-
Bought an old van for 4000 from Peters promising to pay laterwhat is the transactions
-
Company has a following trade credit policy 1/10 N45. If you can borrow from a bank at 9,5% annual rate, would it be beneficial to borrow money and pay off invoices earlier?
-
Given the following exchange rates, which of the multiple-choice choices represents a potentially profitable inter-market arbitrage opportunity? 129.87/$1.1226/$0.00864/ 114.96/ B $0.8908/ (C)...

Study smarter with the SolutionInn App


