Determine the function: h(T) where h(T) = fcdT and C = 11.515 172 1530 0.05 + T
Question:
Determine the function: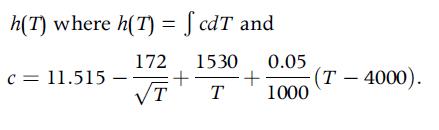
Transcribed Image Text:
h(T) where h(T) = fcdT and C = 11.515 172 1530 0.05 + T T + (T-4000). 1000
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
hT fedT 11515172705 15307 ed...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
Determine the voltage transfer function Vo(s)/Vc(s) as a function of s for the network shown in fig p11.3. R1 He
-
Determine the voltage transfer function Vo(s)/Vc(s) as a function of s for the network shown in fig p11.1.
-
Determine the cumulative distribution function for the distribution in Exercise 4-8. Use the cumulative distribution function to determine the probability that a length exceeds 75 millimeters....
-
Name one industry (retail, technology, biopharma, etc) that you find interesting and compelling and state why it attracts your interest. Lookup a public company in that industry - Who is it? Look at...
-
West Coast Utilities had a net profit of $900 million. It has 900 million shares outstanding and paid annual dividends of $0.90 per share. What is the dividend payout ratio?
-
Why are job descriptions important?
-
Describe the role(s) that functional strategies play in implementing the organization's competitive strategy. LO1
-
On March 31, 2014, Kornet Investments paid $4,480,000 for a tract of land and two buildings on it. The plan was to demolish Building 1 and build a new store (Building 3) in its place. Building 2 was...
-
QS 20-4 (Algo) Weighted average: Physical unit flow reconciliation LO P1 Prepare a physical unit flow reconciliation with the following information
-
A developer has purchased a laundromat and an adjacent factory. To keep smoke, which ruins the clothes, out of the dryers the developer can protect the laundromat or install filters on the factorys...
-
Determine the derivative dT/dP of the function T = 32.4P 0.5 12.8P 0.5 .
-
Consult Paragraphs 65-69 of PCAOB Auditing Standard No. 12. Based on your understanding of fraud risk assessment, what three conditions are likely to be present when a fraud occurs (the fraud...
-
Arrow Company processes a food seasoning powder through its Compounding and Packaging departments. In the Compounding Department, direct materials are added at the beginning of the process, and...
-
The 2017 financial statements of LVMH Moet Hennessey Louis Vuitton S.A. are presented in Appendix C at the end of this book. LVMH is a Paris-based holding company and one of the world's largest and...
-
Repeat Problem 10.E1, except design a packed column using 1-in. metal Pall rings. Do the calculations at the top of the column. Approximate HETP for ethanol-water is \(0.366 \mathrm{~m}\). At...
-
We are separating an ethanol-water mixture in a column operating at atmospheric pressure with a total condenser and a partial reboiler. Constant molal overflow (CMO) can be assumed, and reflux is a...
-
Corporate Social Responsibility Problem The Global Reporting Initiative (GRI) is a networkbased organization that has pioneered the development of the world's most widely used sustainability...
-
What is a core competency? Project scope? Project stakeholder?
-
What mass of H2 will be produced when 122 g of Zn are reacted? Zn(s) + 2HCl(aq) ( ZnCl2(aq) + H2(g)
-
Why do we say that the equilibrium constant for the reaction H 2 O H + + OH - (or any other reaction) is dimensionless?
-
Write the expression for the equilibrium constant for each of the following reactions. Write the pressure of a gaseous molecule, X, as P X . a. b. 3Ag*(aq) + PO (aq) = Ag3PO4(s)
-
(a) A favorable entropy change occurs when S is positive. Does the order of the system increase or decrease when S is positive? (b) A favorable enthalpy change occurs when H is negative. Does the...
-
Marigold industries had the following inventory transactions occur during 2020: 2/1/20 Purchase 51 units @ $46 cost/unit 3/14/20 purchase 98 units @ $49 cost/unit 5/1/20 purchase 68 units @ $53...
-
In this investment portfolio simulation, you and the bean counters, will invest and manage a fictitional amount of $ 1 , 0 0 0 , 0 0 0 during next three weeks. The simulation includes two fictitional...
-
Roberson Corporation uses a periodic inventory system and the retail inventory method. Accounting records provided the following information for the 2018 fiscal year: Cost Retail Beginning inventory...

Study smarter with the SolutionInn App


