Question: How to Interpolate. Apply interpolation to the property data in Table B.1 to determine the following properties of saturated liquid H 2 O:A. The specific
How to Interpolate. Apply interpolation to the property data in Table B.1 to determine the following properties of saturated liquid H2O:A. The specific volume at 300.7 KB. The saturation pressure at 282.6 KC. The mass-specific enthalpy at 514 K
Table B.1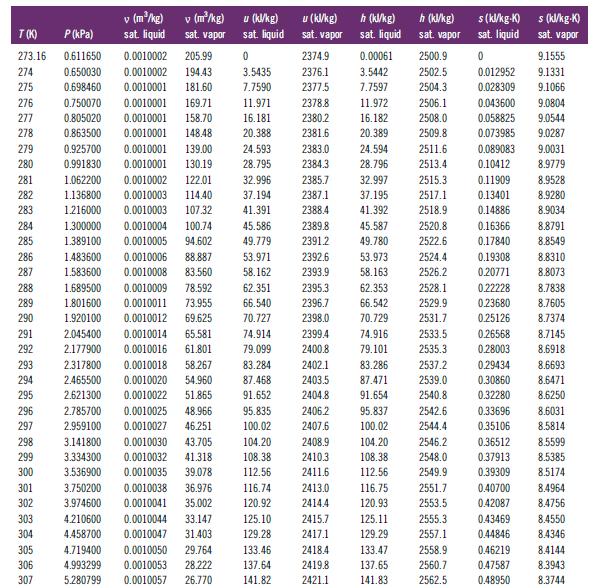
276 277 278 279 280 281 282 283 284 285 286 287 T(K) P (kPa) 273.16 0.611650 274 0.650030 275 0.698460 0.750070 0.0010001 169.71 0.805020 0.0010001 158.70 0.863500 0.0010001 148.48 0.925700 0.0010001 139.00 24.593 0.991830 0.0010001 130.19 28.795 1.062200 0.0010002 122.01 32.996 2385.7 1.136800 0.0010003 114.40 37.194 2387.1 41.391 2388.4 1.216000 0.0010003 107.32 1.300000 0.0010004 100.74 45.586 2389.8 1.389100 0.0010005 94.602 49.779 2391.2 0.0010006 88.887 53.971 0.0010008 83.560 58.162 288 289 290 296 297 298 299 300 1.483600 1.583600 301 302 303 304 305 306 307 1.689500 0.0010009 78.592 1.801600 0.0010011 1.920100 2.045400 291 292 2.177900 293 2.317800 0.0010018 58.267 294 2.465500 0.0010020 54.960 295 2.621300 51.865 0.0010022 0.0010025 48.966 2.785700 2.959100 0.0010027 46.251 3.141800 0.0010030 43.705 3.334300 0.0010032 41.318 3.536900 0.0010035 39.078 0.0010038 36.976 0.0010041 35.002 V (m/kg) sat. liquid 3.750200 3.974600 v (m/kg) sat. vapor 4.210600 4.458700 0.0010002 205.99 0.0010002 194.43 0.0010001 181.60 u (kW/kg) sat. liquid 0 3.5435 7.7590 62.351 66.540 73.955 0.0010012 69.625 70.727 0.0010044 33.147 0.0010047 31.403 11.971 16.181 20.388 0.0010014 65.581 74.914 0.0010016 61.801 79.099 83.284 87.468 91.652 95.835 100.02 104.20 108.38 112.56 4.719400 0.0010050 29.764 4.993299 0.0010053 28.222 5.280799 0.0010057 26.770 116.74 120.92 125.10 129.28 u (kJ/kg) sat. vapor 133.46 137.64 141.82 2374.9 2376.1 2377.5 2378.8 2380.2 2381.6 2383.0 2384.3 2392.6 2393.9 2395.3 2396.7 2398.0 2399.4 2400.8 2402.1 2403.5 2404.8 h (kJ/kg) sat. liquid 2413.0 2414.4 2415.7 2417.1 2418.4 2419.8 2421.1 0.00061 3.5442 7.7597 11.972 16.182 20.389 45.587 49.780 2506.1 2508.0 2509.8 24.594 2511.6 28.796 2513.4 32.997 2515.3 37.195 2517.1 41.392 2518.9 2528.1 2529.9 2531.7 2533.5 2535.3 2537.2 2539.0 2540.8 2406.2 95.837 2542.6 2407.6 100.02 2544.4 2408.9 2546.2 2410.3 2548.0 2411.6 83.286 87.471 91.654 h (kJ/kg) sat. vapor 104.20 108.38 112.56 2500.9 2502.5 2504.3 116.75 120.93 125.11 129.29 133.47 137.65 141.83 2520.8 2522.6 0.16366 0.17840 53.973 2524.4 0.19308 58.163 2526.2 0.20771 62.353 66.542 70.729 74.916 79.101 2549.9 2551.7 2553.5 2555.3 2557.1 s(kl/kg-K) sat. liquid 0 2558.9 2560.7 2562.5 0.012952 0.028309 0.043600 0.058825 0.073985 0.089083 0.10412 0.11909 0.13401 0.14886 0.22228 0.23680 0.25126 0.26568 0.28003 0.29434 0.30860 0.32280 0.33696 0.35106 0.36512 0.37913 0.39309 0.40700 0.42087 0.43469 0.44846 0.46219 0.47587 0.48950 s (kJ/kg-K) sat. vapor 9.1555 9.1331 9.1066 9.0804 9.0544 9.0287 9.0031 8.9779 8.9528 8.9280 8.9034 8.8791 8.8549 8.8310 8.8073 8.7838 8.7605 8.7374 8.7145 8.6918 8.6693 8.6471 8.6250 8.6031 8.5814 8.5599 8.5385 8.5174 8.4964 8.4756 8.4550 8.4346 8.4144 8.3943 8.3744
Step by Step Solution
3.51 Rating (161 Votes )
There are 3 Steps involved in it
Apply interpolation to find the following properties of saturated liquid water from Table B1 ... View full answer

Get step-by-step solutions from verified subject matter experts


