It is desired to pump water at 50 gal/min from 1 to 3 atm. The water temperature
Question:
It is desired to pump water at 50 gal/min from 1 to 3 atm. The water temperature is 25° C. Determine the minimum power input required, assuming ideal frictionless operation and incompressible flow.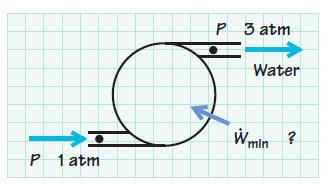
Transcribed Image Text:
P 1 atm P 3 atm J Water W min ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
Known Pumping water to a reservoir 110 m above the first reservoir Find T...View the full answer

Answered By

Cristine kanyaa
I possess exceptional research and essay writing skills. I have successfully completed over 5000 projects and the responses are positively overwhelming . I have experience in handling Coursework, Session Long Papers, Manuscripts, Term papers, & Presentations among others. I have access to both physical and online library. this makes me a suitable candidate to tutor clients as I have adequate materials to carry out intensive research.
4.90+
1538+ Reviews
3254+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
The pump having the characteristics shown in Problem 14.14 is used to pump water from one reservoir to another that is 95 m higher in elevation. The water will flow through a steel pipe that is 0.28...
-
The pump having the characteristics shown in Problem 14.14 was used as a model for a prototype that is to be six times larger. If this prototype operates at 400 rpm, what (a) power; (b) head; and (c)...
-
The pump having the characteristics shown in Problem 14.14 is to be operated at 800 rpm. What discharge rate is to be expected if the head developed is 410 m? Data From Problem 14.14 Performance...
-
IfAUB=AUC and An B=An C, then B = C. Statement-2 AU (BOC) = (AUB) n (AUC)
-
The following adjusted trial balance contains the accounts and balances of Ferrara Company as of December 31, 2011, the end of its fiscal year. (1) Prepare the December 31, 2011, closing entries for...
-
Choose four different lodging properties in your area. Based on your knowledge of these properties, assign a classification or rating based on the standardized system described in this chapter....
-
Restoring self-control while intoxicated. Refer to the Experimental and Clinical Psychopharmacology (Feb. 2005) study of restoring self-control while intoxicated, presented in Exercise 10.40 (p....
-
Using the earnings data developed in E3-1, and assuming that this was the fiftieth week of employment for Jolly and the previous earnings to date were $99,800, prepare the journal entries for the...
-
Edward Hughes has just won the state lottery and has the following three payout options for after-tax prize money 1. $160,000 per year at the end of each of the next six years 2. $300,000 (lump sum)...
-
In this mini-case you will perform some procedures required as a part of audit planning. For ease your audit manager has already organized the workpapers and completed several of the required...
-
In a refrigerator, saturated liquid R-134a (a refrigerant) is throttled from an initial temperature of 305 K to a final pressure of 80 kPa. Determine the final temperature and specific volume of the...
-
Define the isentropic efficiency of a pump/compressor.
-
Turner Corporation uses the calendar year as its tax year. It purchases and places into service $1.97 million of property during 2018 to use in its business: What is Turner's total depreciation...
-
A parent acquires all of the stock of a subsidiary for $40 million in cash. The subsidiarys books report the following account balances at the date of acquisition (in trial balance format)....
-
1. Given: The sign for the Inn of the Prancing Pony in Bree-yes, it comes in pints-is fixed on the end of a beam of length 5L. If the sigh deflects too much then Gandalf will hit his head when he...
-
Q21) Add positive and negative charges as shown in the diagram below. Use the arrows of the simulation to guide you in drawing continuous electric field lines around and in between the three charges....
-
When 10.1 g CaO is dropped into a styrofoam coffee cup containing 157 g H2O at 18.0C, the temperature rises to 35.8C. Calculate the enthalpy change of the following reaction in kJ/mol CaO. Assume...
-
4-12. Sometimes heterogeneous chemical reactions take place at the walls of tubes in which reactive mixtures are flowing. If species A is being consumed at a tube wall because of a chemical reaction,...
-
The statistical mechanical expression for K P consisted of two general parts. What are these parts, and what energetic degrees of freedom do they refer to?
-
Sue Deliveau opened a software consulting firm that immediately paid $2,000 for a computer. Was this event a transaction for the business?
-
Evaluate each of the following to three significant figures and express each answer in SI units using an appropriate prefix: (a) (684 m)/(43 ms) (b) (28ms)(0.0458 Mm)/(348 mg) (c) (2.68 mm)(426 Mg)
-
he density (mass volume) of aluminum is 5.26 slug/ft 3 . Determine its density in SI units. Use an appropriate prefix.
-
Evaluate each of the following to three significant figures and express each answer in SI units using an appropriate prefix: (a) (212 mN) 2 (b) (52800 ms) 2 (c) [548(10 6 ) 1/2 ms.
-
.Is bankruptcy on the part of the borrower a common risk that frequently interferes with a lenders efforts to work out a defaulted loan through either nonforeclosure means or foreclosure? Discuss.
-
For each of the following, compute the future value: Present Value Years Interest Rate $ 1 , 2 5 0 1 9 1 2 % $ 9 8 , 7 2 7 1 5 1 3 % $ 6 2 5 6 1 2 % $ 1 1 7 , 6 2 2 7 1 6 % 2 . For each of the...
-
Only need help on 4B and 5. Exercise 9-21 Breakeven Planning; Profit Planning (LO 9-2, 9-3] Connelly Inc., a manufacturer of quality electric ice cream makers, has experienced a steady growth in...

Study smarter with the SolutionInn App


