Steam expands is entropically (i.e., at constant entropy s) from 2MPa and 500 K to a final
Question:
Steam expands is entropically (i.e., at constant entropy s) from 2MPa and 500 K to a final state in which the steam has become saturated vapor. What is the temperature of the steam at the final state?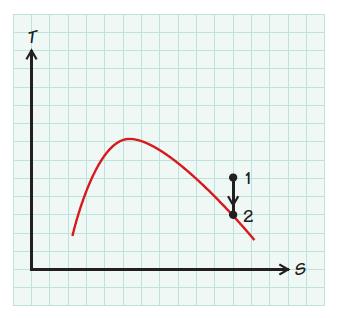
Transcribed Image Text:
T 2 SE
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
saturated vapor at 2 MPa will be greater than or equal to the entropy at the ini...View the full answer

Answered By

Saleem Abbas
Have worked in academic writing for an a years as my part-time job.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
Steam expands is entropically (i.e., at constant entropy s) from 2MPa and 500 K to a final state in which the quality is 0.90. Determine the final-state temperature, pressure, and specific volume. T...
-
A piston-cylinder device contains 0.1 kg of steam at 900 kPa and 320oC. Steam then expands to a final state of 180 kPa and 135oC, doing work. Heat losses from the system to the surroundings are...
-
A cylinder fitted with a piston contains air at 400 K, 1.0 MPa, at which point the volume is 100 L. The air now expands to a final state at 300 K, 200 kPa, and during the process the cylinder...
-
QI: Obtain the closed loop transfer function for the system whose block diagram is shown in fig.(1) 3 Y(s) 2 +. X(s) s+2 s+2 s+2 Fig. (1)
-
In 1995, an analysis of the capital structure of Reebok provided the following results on the cost of capital and firm value. This analysis was based on the 1995 EBIT of $420 million and a tax rate...
-
Joy Don Corp. sells a building to Trifle and Life in exchange for a note. The note specifies a lump sum payment of $300,000 ten years in the future and annual payments (beginning today) of $2,000 at...
-
Suppose a random sample of n = 25 measurements is selected from a population with mean p and standard deviation u. For each of the following values of p and u, give the values of pi and a,. a. p =...
-
Delille Company manufactures both traditional toothpaste and gel toothpaste, with each type of toothpaste produced in separate departments. Three support departments support the production...
-
In your reflection, address the various capital budgeting analysis tools and explain which one you feel is the most efficient and effective tool for companies to use and why. The Reflection body...
-
People in a city are asked if they support a new recycling law. (a) What are the cases? (b) What is the variable and is it quantitative or categorical?
-
Apply interpolation to the property data in Table B.3 to determine the following properties of superheated steam: A. The specific volume at 727 K and 1.5 MPaB. The mass-specific enthalpy at 823 K and...
-
A water heater operating under steady-flow conditions delivers 10 liters/min at 75 C and 370 kPa. The input conditions are 10 C and 379 kPa. What are the corresponding changes in internal energy and...
-
Characterize strong electrolytes versus weak electrolytes versus nonelectrolytes. Give examples of each. How do you experimentally determine whether a soluble substance is a strong electrolyte, weak...
-
A company which manufactures microwaves advertises that 90% of their microwaves are flawless, requiring no adjustments. Their quality control department tests this percentage on a regular basis. On...
-
A new retail store is being planned for a site that contains 40 ft of soft clay (c 0.075 ft2/day, y = 100 pcf). The clay layer is overlain by 15 ft of sand (y = 112 pcf) and is underlain by dense...
-
Perez Bags (PB) is a designer of high-quality backpacks and purses. Each design is made in small batches. Each spring, PB comes out with new designs for the backpack and for the purse. The company...
-
Find a recent (within the last 12 months) article or economic blog related to price fixing, provide an executive summary of the information. Include an APA reference and/or link. How does the fact...
-
A rectangular block of a material with a modulus of rigidity G=90 ksi is bonded to two rigid horizontal plates. The lower plate is fixed, while the upper plate is subjected to a horizontal force P....
-
Refer to case 48. Describe which parts of the design phase Miller should undertake to ensure that the purchased software matches with the business processes that it will use. In case Miller...
-
Prairie Outfitters, Inc., a retailer, accepts paymnent through credit cards. During August, credit card sales amounted to $12,000. The processor charges a 3% fee. Assuming that the credit card...
-
(a) Use the principle of dimensional consistency to show that when Bernoullis equation is written in the form each term has the dimension of pressure. (b) When the equation is written alternatively...
-
A device called a venturi flowmeter can be used to determine the velocity of a flowing fluid by measuring the change in its pressure between two points (Figure P6.35, see on page 290). Water flows...
-
Water flows through a circular pipe having a constriction in diameter from 1 in. to 0.5 in. The velocity of the water just upstream of the constriction is 4 ft/s. By using the result of Problem...
-
A corporate bond that you own at the beginning of the year is worth $930. During the year, it pays $56 in interest payments and ends the year valued at $920. What was your dollar return and percent...
-
Anissa makes custom bird houses in her garage and she buys all her supplies from a local lumber yard. Last year she purchased $4500 worth of supplies and produced 2500 bird houses. She sold all 2500...
-
Critically comment on the following methods that have been used to account for goodwill i)Writting -off the cost of goodwill directly to reserves in the year of acquisition ii) Reporting goodwill at...

Study smarter with the SolutionInn App


