2000 kg of oxygen is in a 1.8 m 3 container maintained at 90 K. Calculate the...
Question:
2000 kg of oxygen is in a 1.8 m3 container maintained at 90 K. Calculate the liquid and vapor masses (kg) and the liquid and vapor volumes (m3). Use the tables in Appendix A.8 for data.
Data From Appendix A.8
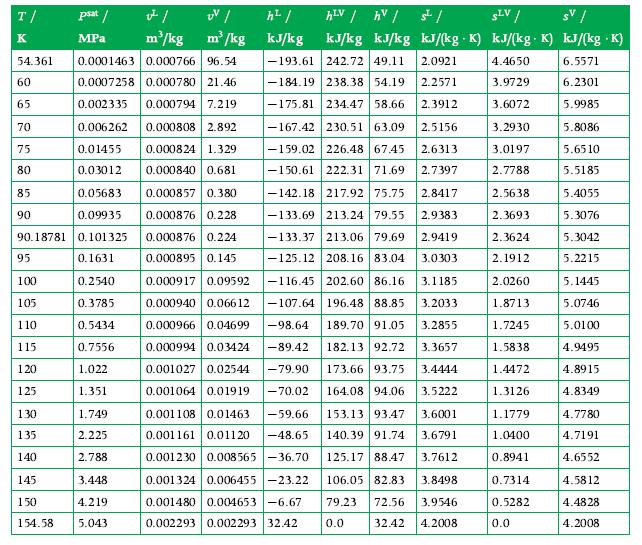
Transcribed Image Text:
T/ K 85 90 54.361 0.0001463 0.000766 96.54 60 0.0007258 0.000780 21.46 65 0.002335 0.000794 7.219 70 0.006262 0.000808 2.892 75 0.01455 0.000824 1.329 80 0.03012 0.000840 0.681 0.05683 0.000857 0.380 0.09935 0.000876 0.228 90.18781 0.101325 0.000876 0.224 0.1631 0.000895 0.145 0.2540 0.3785 0.5434 0.7556 1.022 1.351 95 100 105 110 115 120 125 psat / MPa 130 135 140 145 150 1.749 2.225 2.788 3.448 4.219 154.58 5.043 v²/ UV/ h²/ m³/kg m³/kg kJ/kg hLV / hv / SLV / sv / kJ/kg kJ/kg kJ/(kg K) kJ/(kg K) kJ/(kg .K) 4.4650 6.5571 3.9729 6.2301 3.6072 5.9985 3.2930 5.8086 3.0197 5.6510 2.7788 5.5185 5.4055 5.3076 5.3042 5.2215 5.1445 5.0746 5.0100 4.9495 4.8915 4.8349 4.7780 4.7191 4.6552 4.5812 4.4828 4.2008 0.000917 0.09592 0.000940 0.06612 0.000966 0.04699 0.000994 0.03424 -89.42 -79.90 0.001027 0.02544 0.001064 0.01919 -70.02 0.001108 0.01463 -59.66 0.001161 0.01120 -48.65 0.001230 0.008565-36.70 0.001324 0.006455-23.22 0.001480 0.004653-6.67 0.002293 0.002293 32.42 ملی -193.61 242.72 49.11 2.0921 -184.19 238.38 54.19 2.2571 -175.81 234.47 58.66 2.3912 -167.42 230.51 63.09 2.5156 226.48 67.45 2.6313 -159.02 -150.61 222.31 71.69 2.7397 -142.18 217.92 75.75 2.8417 -133.69 213.24 79.55 2.9383 -133.37 213.06 79.69 2.9419 -125.12 208.16 83.04 3.0303 -116.45 202.60 86.16 3.1185 -107.64 196.48 88.85 3.2033 -98.64 189.70 91.05 3.2855 182.13 92.72 3.3657 173.66 93.75 3.4444 164.08 94.06 3.5222 153.13 93.47 3.6001 140.39 91.74 3.6791 125.17 88.47 3.7612 106.05 82.83 3.8498 79.23 72.56 3.9546 32.42 0.0 4.2008 2.5638 2.3693 2.3624 2.1912 2.0260 1.8713 1.7245 1.5838 1.4472 1.3126 1.1779 1.0400 0.8941 0.7314 0.5282 0.0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
From the table for saturated state in Appendix A8 we have The mass fraction of vap...View the full answer

Answered By

Zablon Gicharu
I am an educator who possesses the requisite skills and knowledge due to interacting with students for an extended period. I provide solutions to various problems in step-by-step explanations, a well-thought approach and an understandable breakdown. My goal is to impart more straightforward methodologies and understanding to students for more remarkable achievements.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Thermodynamics Fundamentals And Engineering Applications
ISBN: 9780521862738
1st Edition
Authors: William C. Reynolds, Piero Colonna
Question Posted:
Students also viewed these Engineering questions
-
A small pharmaceutical firm plans to manufacture a new drug and has hired you as a consultant to design a condenser to remove the drug from a gasvapor mixture. The mixture, which contains 20 mole% of...
-
A small pharmaceutical firm plans to manufacture a new drug and has hired you as a consultant to design a condenser to remove the drug from a gasvapor mixture. The mixture, which contains 20 mole% of...
-
A small pharmaceutical firm plans to manufacture a new drug and has hired you as a consultant to design a condenser to remove the drug from a gasvapor mixture. The mixture, which contains 20 mole% of...
-
(b) (i) What is the smallest natural number n, such that n!> 2" (ii) Prove that n !> 2" is true any natural number greater than or equal that number. (c) Given a finite set of natural numbers ACN....
-
Whole Life Clinic is a VHWO that has three main programs: Drug rehabilitation Alcohol recovery Weight control Unrestricted public support received during the period was $35,000; revenues from...
-
Describe the nature of relationships between variables
-
A random sample of five pairs of observations were selected, one of each pair from a population with mean p,, the other from a population with mean pp The data are shown in the accompanying table....
-
Westinghouse Electric Corporation The following note appears in the Summary of Significant Accounting Policies section of the Annual Report of Westinghouse Electric Corporation. Note 1 (in part):...
-
Down Under Boomerang, Inc., is considering a new 3-year expansion project that requires an initial fixed asset investment of $1.42 million. The fixed asset will be depreciated straight-line to zero...
-
Katie Scarlett is analyzing the 15-unit apartment building for a client for possible purchase. She expanded her analysis from just the DCF (Income) to include the Direct Cap (Income), the Cost and...
-
Consider the system in the figure below. Initially the gas in the cylinder is at high pressure P i and the piston held in place at volume V i with a lock. At time zero the lock is released and the...
-
Imagine turning the system of Exercise 2.13 vertically. Repeat the analysis, which now will involve the height of the piston. Using the same values as in Exercise 2.13(b), with D = 8 cm, plot the...
-
Discuss how Murimi can use her technical skills to devise the strategy. Renita Murimi is a currency overlay manager and market technician who serves institutional investors seeking to address...
-
Idenfity whether the following book - tax adjustments are permanent or temporary differences. ( a ) Federal Income Tax Expense ( b ) Depreciation Expense ( c ) Accrued Compensation ( d ) Dividends...
-
2 . ) Pozycki, LLC has reported losses of $ 1 0 0 , 0 0 0 per year since its founding in 2 0 1 6 . For 2 0 2 3 , Pozycki anticipates a profit of about $ 1 0 0 , 0 0 0 . There are 3 equal members of...
-
Elena is a single taxpayer for tax year 2023. On April 1st, 2022, Elena's husband Nathan died. On July 13, 2023, Elena sold the residence that Elena and Nathan had each owed and used as their...
-
Rodriguez Corporation issues 12,000 shares of its common stock for $56,600 cash on February 20. Prepare journal entries to record this event under each of the following separate situations. 1. The...
-
Problem 3: A large rectangular plate is loaded in such a way as to generate the unperturbed (i.e. far-field) stress field xx = Cy; yy = -C x; Oxy = 0 The plate contains a small traction-free circular...
-
The manager of the BiLo Supermarket in Mt. Pleasant, Rhode Island, gathered the following information on the number of times a customer visits the store during a month. The responses of 51 customers...
-
What is a manufacturing system?
-
A portable music player is sitting in a docking station (Figure P4.31). The docking station has a mass of 500 g and the player, 100 g. Determine the reaction forces at the two supports. Figure P4.31...
-
Two pots of food are being cooked on a solar cooker (Figure P4.32). The smaller pot weighs 4 lb, and the larger pot weighs 9 lb. Also, due to the thermal expansion of the parabolic reflector, a...
-
Find an example of a structure or machine that has several forces acting on it. (a) Make a clear, labeled drawing of it. (b) Estimate the dimensions and the magnitudes and directions of the forces...
-
Your firm is planning to invest in an automated packaging plant. Harburtin Industries is an all - equity firm that specializes in this business. Suppose Harburtin ' s equity beta is 0 . 8 7 , the...
-
Ned Allen opened a medical practice in Los Angeles, California, and had the following transactions during the month of January. (Click the icon to view the January transactions.) Journalize the...
-
do you need more information or are you working on this? Irene Watts and John Lyon are forming a partnership to which Watts will devote one- half time and Lyon will devote full time. They have...

Study smarter with the SolutionInn App


