3 kg of ammonia at its critical state is placed in a constant-volume container, which is then...
Question:
3 kg of ammonia at its critical state is placed in a constant-volume container, which is then cooled until the temperature is 300 K. How a much energy was transferred as heat from the ammonia in this process, and what tank volume is occupied by liquid at the end of the process? Use the tables in Appendix A.7 for data.
Data From Appendix A.7
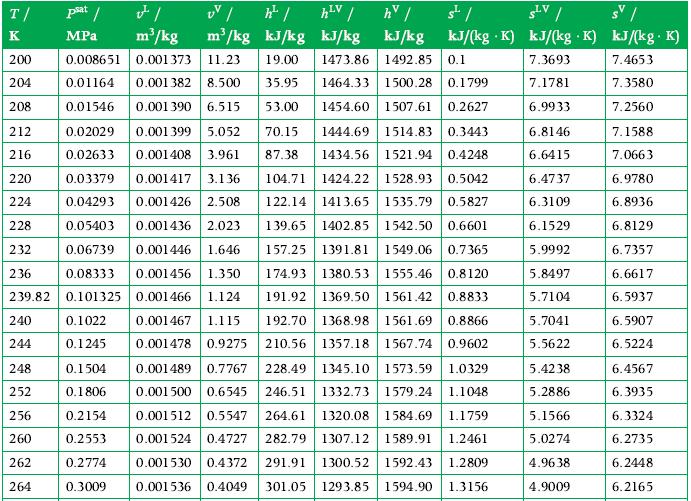
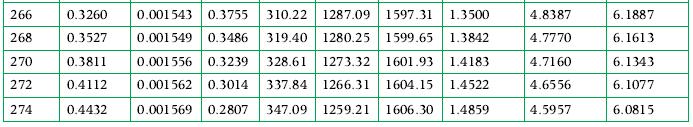
Transcribed Image Text:
T/ K UV / ¹² / of / m³/kg MPa m³/kg kJ/kg 200 0.008651 0.001373 11.23 19.00 204 0.01164 0.001 382 8.500 35.95 208 0.01546 0.001 390 6.515 53.00 212 0.02029 0.001 399 5.052 70.15 1444.69 1514.83 0.3443 216 0.02633 0.001408 3.961 87.38 1434.56 1521.94 0.4248 220 224 228 232 0.03379 0.001417 3.136 104.71 1424.22 1528.93 0.5042 0.04293 0.001426 2.508 122.14 1413.65 1535.79 0.5827 0.05403 0.001436 2.023 139.65 1402.85 1542.50 0.6601 0.06739 0.001446 1.646 157.25 1391.81 1549.06 0.7365 236 0.08333 0.001456 1.350 174.93 1380.53 1555.46 0.8120 239.82 0.101325 0.001466 1.124 191.92 1369.50 1561.42 0.8833 240 0.1022 0.001467 1.115 192.70 1368.98 1561.69 0.8866 0.1245 210.56 1357.18 1567.74 0.9602 244 0.001478 0.9275 0.001489 0.7767 228.49 1345.10 1573.59 1.0329 0.001500 0.6545 246.51 1332.73 1579.24 1.1048 0.001512 0.5547 264.61 1320.08 1584.69 1.1759 0.001524 0.4727 282.79 1307.12 1589.91 1.2461 0.001530 0.4372 291.91 1300.52 1592.43 1.2809 0.001536 0.4049 301.05 1293.85 1594.90 1.3156 248 252 256 260 262 264 0.1504 0.1806 hv / "/ sv/ kJ/kg kJ/(kg K) kJ/(kg K) kJ/(kg. K) 7.3693 7.4653 7.1781 7.3580 6.9933 7.2560 6.8146 7.1588 6.6415 7.0663 6.4737 6.3109 6.1529 5.9992 5.8497 5.7104 5.7041 5.5622 0.2154 0.2553 0.2774 0.3009 hLV | kJ/kg 1473.86 1492.85 0.1 1464.33 1500.28 0.1799 1454.60 1507.61 0.2627 SLV 5.4238 5.2886 5.1566 5.0274 4.9638 4.9009 6.9780 6.8936 6.8129 6.7357 6.6617 6.5937 6.5907 6.5224 6.4567 6.3935 6.3324 6.2735 6.2448 6.2165
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The energy balance for the iso...View the full answer

Answered By

Swarnim Raut
I tutored few juniors of mine during my Mechanical Engineering and my high school. I have the ability to explain difficult concepts into simple manner. My love for Mechanical Engineering has allowed me to get in depth knowledge of the subject. I like to learn and share it with others.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Thermodynamics Fundamentals And Engineering Applications
ISBN: 9780521862738
1st Edition
Authors: William C. Reynolds, Piero Colonna
Question Posted:
Students also viewed these Engineering questions
-
Please need HELP ASAP! FOLLOW THE INSTRUCTIONS!! Problem 3: Presented below is information related to Macaw Corp., for the year 2019. Net sales Cost of goods sold Selling expenses Administrative...
-
Propane is in a 5 m 3 container as a saturated vapor at 300 K. The sealed tank is then cooled to 260 K by fresh snow. What are the pressure (Pa) and liquid volume in this 260 K state, and how much...
-
One kilogram of R134a is heated in a constant volume container from the critical point to 450 K. How much energy is transferred as heat to the fluid? Use the diagrams or data in Appendix A.4. Data...
-
Design a plan to research and select a new or used automobile.
-
The pre-closing, year-end trial balance for a capital projects fund of the city of Rochester as of December 31, 2019, follows: Required 1. Prepare closing entries as of December 31, 2019. 2. Prepare...
-
What are the steps to follow in questionnaire design?
-
Consider dot plots a and b shown above. In which plot is the difference between the sample means small relative to the variability within the sample observations? Justify your answer.
-
Refer to the Real Estate data, which report information on homes sold in the Goodyear, Arizona, area during the last year. a. The mean selling price (in $ thousands) of the homes was computed earlier...
-
Although investing all at once works best when stock prices are rising, dollar-cost averaging can be a good way to take advantage of a fluctuating market. Dollar-cost averaging is an investment...
-
What Happens When You Dont Deliver on Your Promises Web: www.clearly.ca Facebook: Clearly Canadian If a new product or service seems like the perfect option to solve a problem or capitalize on an...
-
A free-piston engine under development consists of a small piston in a cylinder. Each end of the cylinder behaves like a two-stroke engine, in which the following sequence of processes occurs...
-
Determine the equivalents of 1 m, 1 kg, 1 N, 1 J, and 1 W in a Systme Unis in which both k N and k G are unity, the speed of light is unity, and the second is the only primary unit. Choose a suitable...
-
On July 1, K. Resser opened Ressers Business Services. Ressers accountant listed the following chart of accounts: Cash Supplies Prepaid Insurance Equipment Furniture and Fixtures Accounts Payable K....
-
A pistoncylinder device contains 0.85 kg of refrigerant-134a at 210 oC. The piston that is free to move has a mass of 12 kg and a diameter of 25 cm. The local atmospheric pressure is 88 kPa. Now,...
-
3.3. Using the BEMT, show the effect of increasing linear twist on the variations in inflow, thrust, induced power, profile power, and lift coefficient across the span of a rotor with four blades of...
-
By uploading this work, I attest that the work contained herein is solely my own, that I only used the given equation sheet as a reference, and that I have not received any information from anyone...
-
Demand for patient surgery at Washington General Hospital has increased steadily in the past few years, as seen in the following table: ...
-
Explain product analysis
-
a. Compute the mean of the following sample values: 1.3, 7.0, 3.6, 4.1, 5.0. b. Show that (x x) = 0.
-
Evenflow Power Co. is considering a new project that is a little riskier than the current operations of the company. Thus, management has decided to add an additional 1.5% to the company's overall...
-
Michael Phelps won a record-setting 8 gold medals at the 2008 Beijing Olympics. Now imagine if Phelps had competed in a pool filled with pancake syrup. Would you expect his race times to increase,...
-
The fuel tank on a sport-utility wagon holds 14 gal of gasoline. How much heavier is the automobile when the tank is full compared to when it is empty?
-
The pressure at the bottom of an 18-ft-deep storage tank for gasoline is how much greater than at the top? Express your answer in the units of psi.
-
3 . Accounting.. How does depreciation impact financial statements, and what are the different methods of depreciation?
-
NEED THIS EXCEL TABLE ASAP PLEASE!!!! Presupuesto Operacional y C lculo del COGS Ventas Proyectadas: Ventas Proyectadas: $ 4 5 0 , 0 0 0 Precio por unidad: $ 4 5 0 Unidades vendidas: 4 5 0 , 0 0 0 4...
-
The wash sale rules apply to disallow a loss on a sale of securities_______? Only when the taxpayer acquires substantially identical securities within 30 days before the sale Only when the taxpayer...

Study smarter with the SolutionInn App


