Question: Propane is in a 5 m 3 container as a saturated vapor at 300 K. The sealed tank is then cooled to 260 K by
Propane is in a 5 m3 container as a saturated vapor at 300 K. The sealed tank is then cooled to 260 K by fresh snow. What are the pressure (Pa) and liquid volume in this 260 K state, and how much energy was transferred as heat from the propane? Use the tables in Appendix A.6 for data.
Data From Appendix A.6
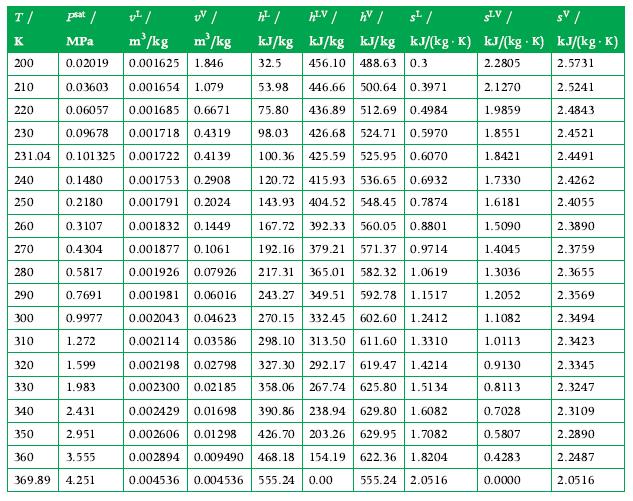
T/ K 200 210 0.03603 0.001654 1.079 220 0.06057 0.001685 0.6671 230 0.09678 0.001718 0.4319 231.04 0.101325 0.001722 0.41 39 0.1480 0.001753 0.2908 0.001791 0.2024 0.2180 0.3107 0.001832 0.1449 0.4304 0.001877 0.1061 0.5817 0.001926 0.07926 0.001981 0.06016 0.7691 0.9977 0.002043 0.04623 1.272 0.002114 0.03586 240 250 260 270 280 290 300 310 psat / v/ UV/ MPa m/kg m/kg 0.02019 0.001625 1.846 320 330 1.599 1.983 2.431 2.951 3.555 369.89 4.251 340 350 360 h / kJ/kg 32.5 HLV / AV / kJ/kg kJ/kg 456.10 488.63 0.3 53.98 446.66 500.64 0.3971 75.80 436.89 512.69 0.4984 98.03 426.68 524.71 0.5970 100.36 425.59 525.95 0.6070 s/ SLV/ sv/ . kJ/(kg. K) kJ/(kg K) kJ/(kg. K) 2.2805 2.5731 2.5241 2.4843 2.4521 2.4491 120.72 415.93 536.65 0.6932 143.93 404.52 548.45 0.7874 167.72 392.33 560.05 0.8801 192.16 379.21 571.37 0.9714 217.31 365.01 582.32 1.0619 243.27 349.51 592.78 1.1517 270.15 332.45 602.60 1.2412 298.10 313.50 611.60 1.3310 327.30 292.17 619.47 1.4214 358.06 267.74 625.80 1.5134 0.002198 0.02798 0.002300 0.02185 0.002429 0.01698 390.86 238.94 629.80 1.6082 0.002606 0.01298 426.70 203.26 629.95 1.7082 0.002894 0.009490 468.18 154.19 622.36 1.8204 0.004536 0.004536 555.24 0.00 555.24 2.0516 2.1270 1.9859 1.8551 1.8421 1.7330 1.6181 1.5090 1.4045 1.3036 1.2052 1.1082 1.0113 0.9130 0.8113 0.7028 0.5807 0.4283 0.0000 2.4262 2.4055 2.3890 2.3759 2.3655 2.3569 2.3494 2.3423 2.3345 2.3247 2.3109 2.2890 2.2487 2.0516
Step by Step Solution
3.52 Rating (152 Votes )
There are 3 Steps involved in it
To solve this problem we will use the following steps Determine the initial state of propane at 300 K Calculate the final state of propane at 260 K as... View full answer

Get step-by-step solutions from verified subject matter experts


