Calculate the density of gaseous CO 2 at each of the following states:a. 10 bar, 290 K,b.
Question:
Calculate the density of gaseous CO2 at each of the following states:a. 10 bar, 290 K,b. 30 bar, 333 K,c. 100 bar, 410 K,Using the perfect gas model. Compare the values obtained with the values listed in the tables in Appendix A.9. What can you infer?
Data From Appendix A.9
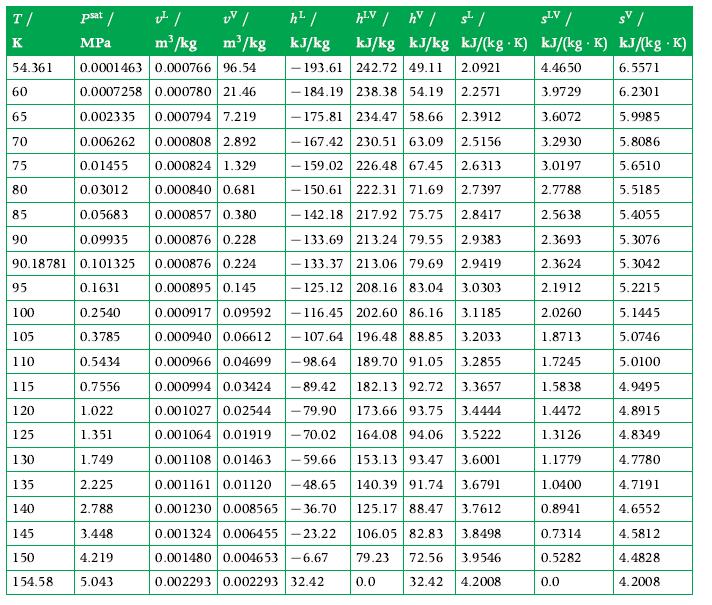
Transcribed Image Text:
T/ K UV/ m³/kg m³/kg 54.361 0.0001463 0.000766 96.54 0.0007258 0.000780 21.46 0.002335 0.000794 7.219 0.000808 2.892 0.000824 1.329 0.006262 0.01455 0.03012 0.000840 0.681 0.05683 0.000857 0.380 0.09935 0.000876 0.228 90.18781 0.101325 0.000876 0.224 0.1631 0.000895 0.145 60 65 70 75 80 85 psat / MPa 90 95 100 0.2540 105 0.3785 110 0.5434 115 0.7556 120 1.022 125 1.351 130 1.749 135 2.225 140 2.788 145 3.448 150 4.219 154.58 5.043 h² / kJ/kg hLV / hv / 5¹/ SLV / SV/ kJ/kg kJ/kg kJ/(kg K) kJ/(kg. K) kJ/(kg .K) 4.4650 6.5571 3.9729 6.2301 3.6072 5.9985 3.2930 5.8086 3.0197 5.6510 2.7788 5.5185 2.5638 5.4055 2.3693 5.3076 2.3624 5.3042 2.1912 5.2215 2.0260 5.1445 1.8713 5.0746 1.7245 5.0100 1.5838 4.9495 1.4472 4.8915 1.3126 4.8349 1.1779 4.7780 1.0400 4.7191 0.8941 4.6552 0.7314 4.5812 0.5282 4.4828 4.2008 193.61 242.72 49.11 2.0921 184.19 238.38 54.19 2.2571 175.81 234.47 58.66 2.3912 - 167.42 230.51 63.09 2.5156 -159.02 226.48 67.45 2.6313 -150.61 222.31 71.69 2.7397 -142.18 217.92 75.75 2.8417 2.9383 -133.69 213.24 79.55 133.37 213.06 79.69 2.9419 -125.12 208.16 83.04 3.0303 0.000917 0.09592 -116.45 202.60 86.16 3.1185 0.000940 0.06612 -107.64 196.48 88.85 3.2033 182.13 92.72 3.3657 0.000966 0.04699 -98.64 189.70 91.05 3.2855 0.000994 0.03424 -89.42 0.001027 0.02544 - 79.90 0.001064 0.01919 - 70.02 173.66 93.75 3.4444 164.08 94.06 3.5222 153.13 93.47 3.6001 0.001108 0.01463 -59.66 0.001161 0.01120 -48.65 3.6791 140.39 91.74 0.001230 0.008565-36.70 125.17 88.47 3.7612 0.001324 0.006455-23.22 0.001480 0.004653-6.67 0.002293 0.002293 32.42 106.05 82.83 3.8498 79.23 72.56 3.9546 0.0 32.42 4.2008 0.0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (7 reviews)
To calculate the density of gaseous CO2 at each of the given states using the perfect gas model we c...View the full answer

Answered By

Monette Taban
I am currently studying Computer Science Engineering, Due to my interest in programming languages and coding, I am interesetd on Technology so I search about it read about different types of technologies, I think my this habbis will help me to solve problems of students and that is why I am signing as a question answer expert.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Thermodynamics Fundamentals And Engineering Applications
ISBN: 9780521862738
1st Edition
Authors: William C. Reynolds, Piero Colonna
Question Posted:
Students also viewed these Engineering questions
-
In order to test the applicability of the ideal gas equation of state to calculate the density of saturated steam, compare the specific volume of saturated steam obtained from the steam table with...
-
(a) Calculate the density of the atmosphere at the surface of Mars (where the pressure is 650 Pa and the temperature is typically 253 K, with a CO 2 atmosphere), Venus (with an average temperature of...
-
Calculate the density of states g (E) for the free-electron model of a metal if E = 7.0eV and V = 1.0 cm3. Express your answer in units of states per electron volt.
-
Let be an arbitrary operation in Problems 5259. Describe the operation for each problem. 5038; 70 2= 9; 901 = 10; 8 0 2 = 10; -
-
If a potential investments internal rate of return is above the companys hurdle rate, should the investment be made?
-
I'm telling you the average is about $18.00, said Maya Stafford, the head server at the Mountain Grill. Maya and Raktida were talking about the check average achieved by the Grill on the previous two...
-
You can gain valuable insights into international business by examining how countries compare to each other. Various research groups and international agencies systematically examine economic,...
-
On October 1, 2014, Daster Corporation declared a $50,000 cash dividend to be paid on December 15 to shareholders of record on November 1. Required Record the events occurring on October 1 and...
-
Locate the annual report for Lowes and Home Depot. These can be obtained from either the company websites or from SEC.gov. Submit a paper providing the following: Provide an analysis of the two...
-
Visit the web page of one of the associations listed under Careers in Operations Management and briefly list the targeted members and the services that they provide to their members.
-
Propane is in a 5 m 3 container as a saturated vapor at 300 K. The sealed tank is then cooled to 260 K by fresh snow. What are the pressure (Pa) and liquid volume in this 260 K state, and how much...
-
Consider each matrix as the augmented matrix of a linear system. State in words the next two elementary row operations that should be performed in the process of solving the system. 1-6 4 2 -7 0 0...
-
In 1996, the Bureau of Consumer Protection of the Federal Trade Commission (FTC) conducted a study to investigate the accuracy of electronic checkout scanners. They inspected the pricing accuracy of...
-
Over the past 40 years, union membership has declined, and it continues to do so. Instead, many companies are turning to alternative dispute resolution. We know one of the best union avoidance...
-
Article "A Leader's Journey" by Pamela Kruger Photographs by Nigel Dickson. For this discussion, let's try and unpack the key factors that led to his transformation. 1. What are your key takeaways...
-
Describe the collaborative roles of the team leader and the team coach in helping a group of people come together to form a team. Recommend strategies for Alex as team leader to use in helping to...
-
a. Complete the table with all marginal totals and cell counts. b. Calculate the following probabilities. i. For a male to be a Republican. ii. For a voter to be female. iii. For a voter to be either...
-
1. Will the Coronavirus Pandemic Make Working from Home the New Normal?" Address the following below. Define the problem described in this case. What are the management, organization, and technology...
-
List some disadvantages of providing all city data in an open, accessible format.
-
Express these numbers in standard notation. a. 2.87 10-8 b. 1.78 1011 c. 1.381 10-23
-
A world-class runner can run half a mile in a time of 1 min and 45 s. What is the runners average speed in m/s?
-
One U.S. gallon is equivalent to 0.1337 ft 3 , 1 ft is equivalent to 0.3048 m, and 1000 L are equivalent to 1 m 3 . By using those definitions, determine the conversion factor between gallons and...
-
A passenger automobile is advertised as having a fuel economy rating of 29 mi/gal for highway driving. Express the rating in the units of km/L.
-
Suppose Universal Forests current stock price is $59.00 and it is likely to pay a $0.57 dividend next year. Since analysts estimate Universal Forest will have a 13.8 percent growth rate, what is its...
-
ABC Company engaged in the following transaction in October 2 0 1 7 Oct 7 Sold Merchandise on credit to L Barrett $ 6 0 0 0 8 Purchased merchandise on credit from Bennett Company $ 1 2 , 0 0 0 . 9...
-
1. Use the Excel file Asset Allocation Data to determine the following: a.Variances for the individual assets b. Standard deviations for the individual assets c.Covariances between each pair of...

Study smarter with the SolutionInn App


