Question: A soap bubble is a system consisting of two subsystems. Subsystem (f) is the thin film and subsystem (g) is the gas enclosed inside the
A soap bubble is a system consisting of two subsystems. Subsystem (f) is the thin film and subsystem (g) is the gas enclosed inside the film. The surrounding air is a thermal bath. The equilibrium is characterised by the minimum of the free energy F of the system. The differential of the free energy dF reads,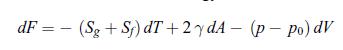
where A is the surface area of the soap film and V the volume of the bubble. The parameter γ is called the surface tension. It characterises the interactions at the interface between the liquid and the air. Since the soap film has two such interfaces, there is a factor 2 in front of the parameter γ. The surface tension γ is an intensive variable that plays an analogous role for a surfacic system as the pressure p for a volumic system. However, the force due to pressure of a gas is exerted outwards whereas the force due to the surface tension is exerted inwards. This is the reason why the signs of the corresponding two terms in dF differ. The term p − p0 is the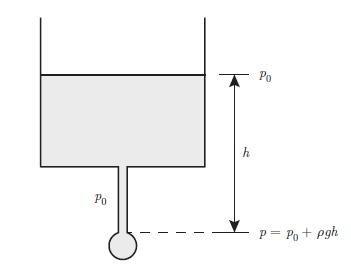
Principle of a setup that could be used to estimate the influence of surface tension on the pressure inside a liquid drop. The container is wide enough so that when a drop is forming the change in height of the liquid is negligible. The system is in a thermal bath at constant temperature T. pressure difference between the pressure p inside the bubble and the atmospheric pressure p0. Consider the bubble to be a sphere of radius r and show that,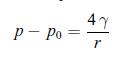
dF = (Sg + Sf) dT +2y dA- (p- po) dv ==
Step by Step Solution
3.39 Rating (171 Votes )
There are 3 Steps involved in it
ANSWER p p0 2r To derive this equation we start with the definition of pressure as force per unit ar... View full answer

Get step-by-step solutions from verified subject matter experts


