Question: Assume that a binary mixture and its components can be modeled using the van der Waals equation of state, both for the liquid and the
Assume that a binary mixture and its components can be modeled using the van der Waals equation of state, both for the liquid and the vapor phase. Assume also that the mixture equation of state parameters can be calculated using so-called van der Waals one-fluid mixture rules,
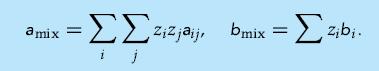
(a) Derive the fugacity coefficient for component i in the mixture and show that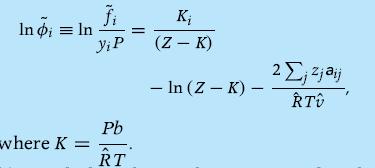
(b) Similarly, obtain the equation for the activity coefficient of component i, γi.
- .iza amix = bmix
Step by Step Solution
3.59 Rating (174 Votes )
There are 3 Steps involved in it
a The fugacity coefficient of a component i in a mix... View full answer

Get step-by-step solutions from verified subject matter experts


