Question: We consider a substance that can be either in a gaseous phase inside a rigid container or in an absorbed phase on the surface of
We consider a substance that can be either in a gaseous phase inside a rigid container or in an absorbed phase on the surface of a substrate inside it. We analyse the equilibrium reached by the substance in the gaseous and adsorbed phases. The entire system is at a fixed temperature T. We assume that when the substance is adsorbed, it acquires a magnetisation M. The reason whether and why this might happen has been the subject of much research [132]. The gas has no magnetisation. Use relation (8.58) for the pressure dependence of the chemical potential of the gaseous phase and assume that the chemical potential of the adsorbed phase is independent of pressure. Find the dependence on magnetic induction field B of the pressure p.
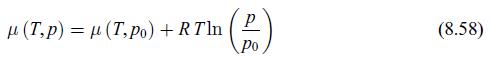
Step by Step Solution
3.48 Rating (155 Votes )
There are 3 Steps involved in it
The relation 858 states that the chemical potential of a gas is given by RT lnpp where is the standa... View full answer

Get step-by-step solutions from verified subject matter experts


