The densities (g/mL) of several substances are: acetic acid, 1.05; CCl 4 , 1.59; S, 2.07; Li,
Question:
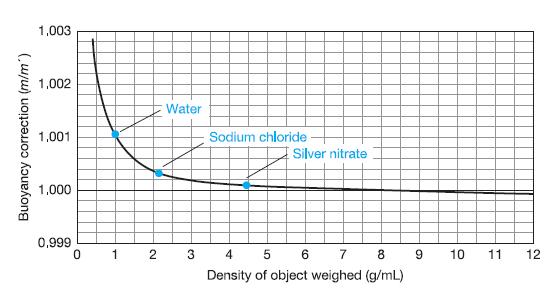
The densities (g/mL) of several substances are: acetic acid, 1.05; CCl4, 1.59; S, 2.07; Li, 0.53; Hg, 13.5; PbO2, 9.4; Pb, 11.4; Ir, 22.5. From Figure 2-9, predict which substances will have the smallest and largest buoyancy corrections.
Figure 2-9
Transcribed Image Text:
1,003 1,002 Water 1,001 Sodium chloride Silver nitrate 1,000 0,999 1 3 4 5 6 10 11 12 Density of object weighed (g/mL) Buoyancy correction (m/m') 2.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (9 reviews)
Buoyancy correction factor m can be found as m 1 m is reading of mass balance da is density of air 0...View the full answer

Answered By

Abhinav Gupta
i am currently working on chegg as chemistry expert since 2018 . I have maintained a C.F. score of minimum 75% since the time I have started solving questions. I have also worked on topper tutors for 1 year for 2017 to 2018 with average 4 star ratings.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Related Video
Density helps us predict whether something will float. Density is super important to consider when building things like ships and submarines. The experiment in the video examines the water density by comparing a glass of water containing sugar with a simple glass of water
Students also viewed these Engineering questions
-
Acetic acid, CH3COOH, is contained in vinegar. Suppose acetic acid was formed from its elements, according to the following equation: 2C(graphite) + 2H2(g) + O2(g) CH3COOH(l) Find the enthalpy...
-
Ka for acetic acid is 1.7 10-5 at 25C. A buffer solution is made by mixing 52.1 mL of 0.122 M acetic acid with 46.1 mL of 0.182 M sodium acetate. Calculate the pH of this solution at 25C after the...
-
The distinctive odor of vinegar is due to acetic acid, CH3COOH, which reacts with sodium hydroxide in the following fashion: If 3.45 mL of vinegar needs 42.5 mL of 0.115 M NaOH to reach the...
-
LOCATE APPROPRIATE CPT CODES ICD-10-CM (CPT) FOR PROCEDURES BELOW- Outpatient procedures only 1. INCISION AND DRAINAGE OF A CYST --- 2. DEBRIDEMENT - 3. SIMPLE REPAIR OF A SUPERFICIAL WOUND- 4....
-
Toby Power, a fellow Illinois resident, is a new client. Prior to her telephone call this morning (late November), all you really knew about Toby was that for the past five years she has been earning...
-
Presented below is an aging schedule for Joplin Company. At December 31, 2007, the unadjusted balance in Allowance for Doubtful Accounts is a credit of $8,000. | Instructions (a) Journalize and post...
-
How should material seasonal variations in revenue be reflected in interim financial statements? LO3 a. The seasonal nature should be disclosed, and the interim report should be supplemented with a...
-
Can Marta sue Chelene for fraudulent misrepresentation? Why or why not? What element(s) might be lacking? Chelene had been a caregiver for Martas eighty-year-old mother, Janis, for nine years....
-
BSBFIM501 Q3, Please help I have been struggling with this subject! Question 3 - Record keeping a) Go to business.gov.au and read the section 'Record keeping'. On what does the record keeping...
-
Ruby-Star Incorporated is considering two different vendors for one of its top-selling products which has an average weekly demand of 50 units and is valued at $75 per unit. Inbound shipments from...
-
Pentane (C 5 H 12 ) is a liquid with a density of 0.626 g/mL near 25C. Find the true mass of pentane when the mass in air is 14.82 g. Assume air density = 0.001 2 g/mL.
-
What do the symbols TD and TC mean on volumetric glassware?
-
a. Read all the instructions first; then you install the printer program. b. Read all the instructions first, and then install the printer program. In the above sentence pairs, choose the one that...
-
"New Push Ties Cost of Drugs to How Well they Work," by Loftus, and "Pay-For-Performance is No Miracle Cure," by Pauly; the authors make an argument that tying the price of a drug to its...
-
Entrepreneurs/entrepreneurship is a driving force of the U.S. economy. It has also provided great personal wealth to a host of entrepreneurs, past and present, who have dared to risk all for a dream....
-
Select a topic (the purpose) of your choice and persuade people (the audience) o As a starting point for topic ideas, consider selling a product, advertising an event, or sharing a thoughtful article...
-
What is java?
-
Identify each of the following costs, incurred monthly by Furman Flower Cart, as fixed, variable, or mixed. Explain your reasoning. Bouquets Sold 2,000 4,000 6,000 $ 5,000 $ 6,000 $12,000 $15,000 $...
-
During the year land was revalued and the surplus reported as Revaluation surplus; and an asset costing 80,000, written down to 38,000, was sold for 40,000. Identify the cost of any non-current...
-
Vernier scale. The figure below shows a scale found on instruments such as a micrometer caliper used for accurately measuring dimensions of objects. The lower scale slides along the upper scale and...
-
Controlling the appearance of a graph. Figure 3-3 requires gridlines to read buret corrections. In this exercise, you will format a graph so that it looks like Figure 3-3. Follow the procedure in...
-
Why do we use quotation marks around the word true in the statement that accuracy refers to how close a measured value is to the true value?
-
As a long-term investment at the beginning of the 2018 fiscal year, Florists International purchased 25% of Nursery Supplies Inc.'s 18 million shares for $66 million. The fair value and book value of...
-
Javier is currently paying $1,200 in interest on his credit cards annually. If, instead of paying interest, he saved this amount every year, how much would he accumulate in a tax-deferred account...
-
Your company is considering the purchase of a fleet of cars for $195,000. It can borrow at 6%. The cars will be used for four years. At the end of four years they will be worthless. You call a...

Study smarter with the SolutionInn App


