Calculate the values of specific volume, specific internal energy, and specific enthalpy for water in vaporliquid equilibrium
Question:
Calculate the values of specific volume, specific internal energy, and specific enthalpy for water in vapor–liquid equilibrium at 180°C if its quality is 0.2. Use the tables in Appendix A.3 for data.
Data From Appendix A.3
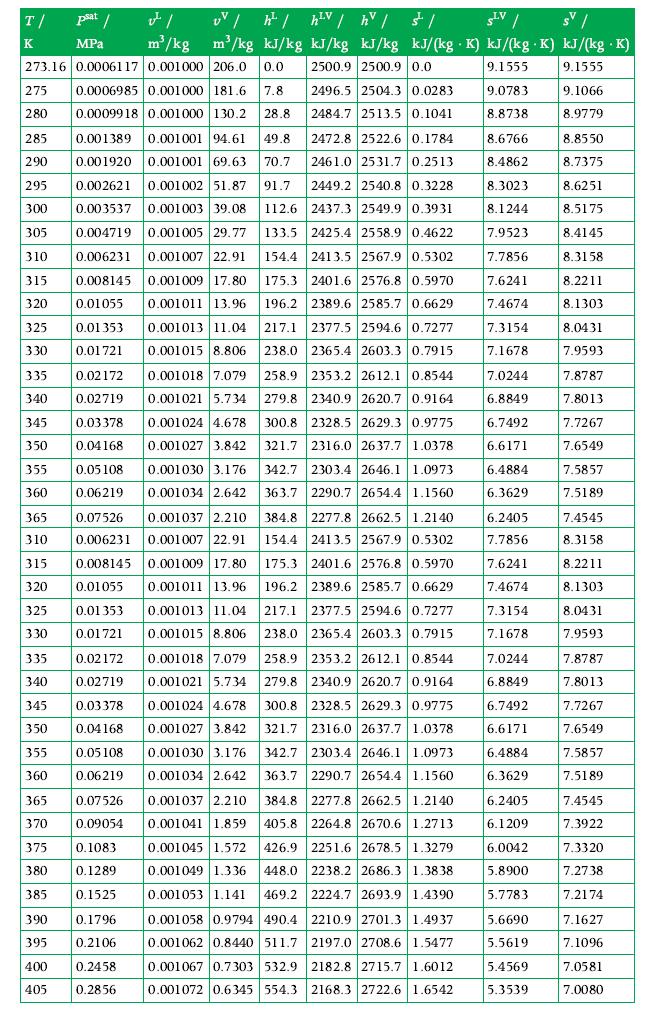
Transcribed Image Text:
T/ psat / MPa K v m³/kg 273.16 0.0006117 0.001000 206.0 0.0 0.0006985 0.001000 181.6 7.8 0.0009918 0.001000 130.2 28.8 335 340 345 350 355 360 UV /h²/h²V / hv / / SLV / sv/ m³/kg kJ/kg kJ/kg kJ/kg kJ/(kg K) kJ/(kg.K) kJ/(kg .K) 2500.9 2500.9 0.0 9.1555 9.1555 9.0783 8.8738 275 280 285 0.001389 0.001001 94.61 49.8 290 0.001920 0.001001 69.63 70.7 295 0.002621 0.001002 51.87 91.7 300 0.003537 0.001003 39.08 112.6 305 0.004719 0.001005 29.77 133.5 310 0.006231 0.001007 22.91 0.001009 17.80 175.3 315 0.008145 320 0.01055 0.001011 13.96 325 0.01 353 0.001013 11.04 330 0.01721 0.001015 8.806 0.02172 0.02719 0.03378 0.04168 0.05108 0.06219 0.07526 0.006231 365 310 325 330 0.001018 7.079 0.001021 5.734 0.001024 4.678 0.001027 3.842 0.001030 3.176 0.001034 2.642 0.001037 2.210 0.001007 22.91 315 0.008145 0.001009 17.80 320 0.01055 0.001011 13.96 0.01 353 0.001013 11.04 0.01721 0.001015 8.806 335 0.02172 0.001018 7.079 0.001021 5.734 340 0.02719 345 0.03378 350 0.04168 355 0.05108 360 0.06219 365 0.07526 0.001024 4.678 0.001027 3.842 0.001030 3.176 0.001034 2.642 370 0.09054 375 0.1083 380 0.1289 385 0.1525 390 0.1796 395 0.2106 400 0.2458 405 0.2856 2496.5 2504.3 0.0283 2484.7 2513.5 0.1041 2472.8 2522.6 0.1784 2461.0 2531.7 0.2513 2449.2 2540.8 0.3228 2437.3 2549.9 0.3931 2425.4 2558.9 0.4622 154.4 2413.5 2567.9 0.5302 2401.6 2576.8 0.5970 196.2 2389.6 2585.7 0.6629 217.1 2377.5 2594.6 0.7277 238.0 2365.4 2603.3 0.7915 258.9 2353.2 2612.1 0.8544 279.8 2340.9 2620.7 0.9164 2328.5 2629.3 0.9775 300.8 321.7 2316.0 2637.7 1.0378 2303.4 2646.1 1.0973 342.7 363.7 2290.7 2654.4 1.1560 2277.8 2662.5 1.2140 384.8 154.4 2413.5 2567.9 0.5302 175.3 2401.6 2576.8 0.5970 196.2 2389.6 2585.7 0.6629 217.1 2377.5 2594.6 0.7277 238.0 2365.4 2603.3 0.7915 258.9 2353.2 2612.1 0.8544 2340.9 2620.7 0.9164 279.8 300.8 2328.5 2629.3 0.9775 321.7 342.7 2303.4 2646.1 1.0973 363.7 2290.7 2654.4 1.1560 2316.0 2637.7 1.0378 384.8 2277.8 2662.5 1.2140 405.8 2264.8 2670.6 1.2713 0.001037 2.210 0.001041 1.859 0.001045 1.572 426.9 2251.6 2678.5 1.3279 0.001049 1.336 0.001053 1.141 469.2 0.001058 0.9794 490.4 2210.9 2701.3 1.4937 0.001062 0.8440 511.7 2197.0 2708.6 1.5477 0.001067 0.7303 532.9 2182.8 2715.7 1.6012 0.001072 0.6345 554.3 2168.3 2722.6 1.6542 448.0 2238.2 2686.3 1.3838 2224.7 2693.9 1.4390 8.6766 8.4862 8.3023 8.1244 7.9523 7.7856 7.6241 7.4674 7.3154 7.1678 7.0244 6.8849 6.7492 6.6171 6.4884 6.3629 6.2405 7.7856 7.6241 7.4674 7.3154 7.1678 7.0244 6.8849 6.7492 6.6171 6.4884 6.3629 6.2405 6.1209 6.0042 5.8900 5.7783 5.6690 5.5619 5.4569 5.3539 9.1066 8.9779 8.8550 8.7375 8.6251 8.5175 8.4145 8.3158 8.2211 8.1303 8.0431 7.9593 7.8787 7.8013 7.7267 7.6549 7.5857 7.5189 7.4545 8.3158 8.2211 8.1303 8.0431 7.9593 7.8787 7.8013 7.7267 7.6549 7.5857 7.5189 7.4545 7.3922 7.3320 7.2738 7.2174 7.1627 7.1096 7.0581 7.0080
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
From the table we can see that the closest temperature to 180C is 1753C The table shows data at inte...View the full answer

Answered By

Brian Kiprono
Taking part in public speeches, blogging, writing essays
0.00
0 Reviews
10+ Question Solved
Related Book For 

Thermodynamics Fundamentals And Engineering Applications
ISBN: 9780521862738
1st Edition
Authors: William C. Reynolds, Piero Colonna
Question Posted:
Students also viewed these Engineering questions
-
Calculate the values of S and DG for each of the following processes at 298 K: H2O(l,298 K) H2O(g, V = 1000. L/mol) H2O(l,298 K) H2O(g, V = 100. L/mol) The standard enthalpy of vaporization for...
-
A 3 ft deep fish pond in the shape of a hemisphere has a volume of 56.549 ft3 when it is full. These data have been collected relating depth to volume in the pond: a. Find the equation of the least...
-
Consider the following hypothesis test: H: 45 H: < 45 A sample of 36 is used. Identify the p-value and state your conclusion for each of the following sample results. Use =.01. a. x = 44 and s...
-
Remove left recursion in the following grammar. Show each step. Hint: First remove direct left recursion. Then the indirect left recursion. A AB | Aab | BA| a Bb | | b
-
Distinguish between accounting and financial reporting for state and local governments and VHWOs for the following issues: 1. Measurement focus and basis of accounting 2. Revenue recognition 3....
-
Engico, Inc. is not alone in its push to diversity. How might you use existing secondary data in your research approach? Where might you find it? What would be most helpful?
-
Two independent random samples are taken from two populations. The results of these samples are summarized in the next table. a. Form a 90% confidence interval for (p, - p2). b. Test H,: (pI - p2) =...
-
For Briggs Company actual sales are $1,200,000 and break-even sales are $900,000. Compute (a) The margin of safety in dollars and (b) The margin of safety ratio.
-
A mutual fund earned an average annual return of 5.5% over the last 5 years. During that time, the average risk-free rate was 1.1% and the average market return was 7.8%. If the fund has beta of...
-
Use the construction in the proof of Theorem 1.45 to give the state diagrams of NFAs recognizing the union of the languages described in a. Exercises 1.6a and 1.6b. b. Exercises 1.6c and 1.6f....
-
Imagine turning the system of Exercise 2.13 vertically. Repeat the analysis, which now will involve the height of the piston. Using the same values as in Exercise 2.13(b), with D = 8 cm, plot the...
-
How much energy transfer as heat (J) is required to make fluid in a constant volume container undergo a change U = 200 kJ/kg?
-
A baby is bouncing in a seat fastened by an elastic cord to a hook on the ceiling. At the top of his bounce, his velocity is instantaneously zero. Is the vector sum of forces exerted on him at that...
-
4) This question concerns the simulation of price trajectories in the Black-Scholes model. We therefore want to simulate price vectors: (St St, Str); where tiit, i=0,1,..., n. The total number of...
-
Consider a pure exchange economy with two goods, (x,y), and two consumers, (1,2). Con- sumers' endowments are e (4,2) and e = (6,6) and their preferences are represented by utility functions: u(x,y)...
-
O The national highways agency releases information on the pro- portion of people not wearing seatbelts, aggregated by city. The data comes from random traffic stops conducted between 8am and 9am on...
-
Ivanka's Budgeted Income Statement You are the accountant for Ivanka Ltd which operates a small mixed business. The following estimates relate to the base year (Year 1): Sales of product A $100 000...
-
1. Implement the function of a XNOR gate by a 2 to 4 decoder. Use logic gates if needed at the output. 2. The following question is to design an octal to binary encoder. a) Write down the truth table...
-
Describe the similarities and differences of qualitative and quantitative variables. Be sure to include the following: a. What level of measurement is required for each variable type? b. Can both...
-
The diameter of a sphere is 18 in. Find the largest volume of regular pyramid of altitude 15 in. that can be cut from the sphere if the pyramid is (a) square, (b) pentagonal, (c) hexagonal, and (d)...
-
Grade 1020 steel has a yield strength of 42 ksi and an elastic modulus of 30 Mpsi. Another grade of steel has a yield strength of 132 ksi. What is its elastic modulus?
-
A steel cable of diameter 3/16 in. is attached to an eyebolt and tensioned to 500 lb (Figure P5.4, see on page 226). Calculate the stress in the cable, and express it in the dimensions psi, ksi, Pa,...
-
When a 120-lb woman stands on a snow-covered trail, she sinks slightly into the snow because the compressive stress between her ski boots and the snow is larger than the snow can support without...
-
Just work out the assignment on your own sheet, you dont need the excel worksheet. Classic Coffee Company Best friends, Nathan and Cody, decided to start their own business which would bring great...
-
Financial information related to the proprietorship of Ebony Interiors for February and March 2019 is as follows: February 29, 2019 March 31, 2019 Accounts payable $310,000 $400,000 Accounts...
-
(b) The directors of Maureen Company are considering two mutually exclusive investment projects. Both projects concern the purchase of a new plant. The following data are available for each project...

Study smarter with the SolutionInn App


