Given the set of VLE experimental data for the non-ideal mixture propane/n-pentane at 344.26 K from B.
Question:
Given the set of VLE experimental data for the non-ideal mixture propane/n-pentane at 344.26 K from B. Sage and W. Lacey, “Phase equilibria in hydrocarbon systems, propanen- pentane system,” Industrial and Engineering Chemistry, 32, (7), 992–996, 1940,
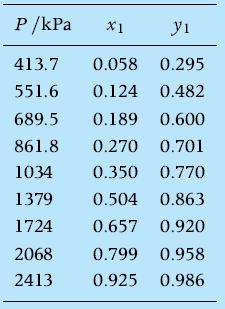
correlate them with the Margules equations and make the T–xy chart at P = 400 kPa under the assumption that the vapor phase obeys the ideal gas law, while the liquid is a non-ideal liquid mixture.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Thermodynamics Fundamentals And Engineering Applications
ISBN: 9780521862738
1st Edition
Authors: William C. Reynolds, Piero Colonna
Question Posted:





