Given the truncated virial equation for calculate the pressure for T = 180 K and v =
Question:
Given the truncated virial equation for
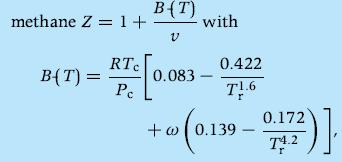
calculate the pressure for T = 180 K and v = 0.03 m3/kg and calculate the specific volume for P = 0.3 MPa and T = 320 K. Compare the values with the values obtained with the ideal gas equation of state. Indicate the points corresponding to this states in the P-h diagram of Appendix A.5 and highlight the thermodynamic region where the ideal gas assumption applies. The critical temperature and pressure of methane are Tc?= 190.6 K and Pc = 4.60 MPa, respectively, the molecular weight M? = 16.04 kg/kmol, the acentric factor ? = 0.0114, and Tr?= T/Tc.
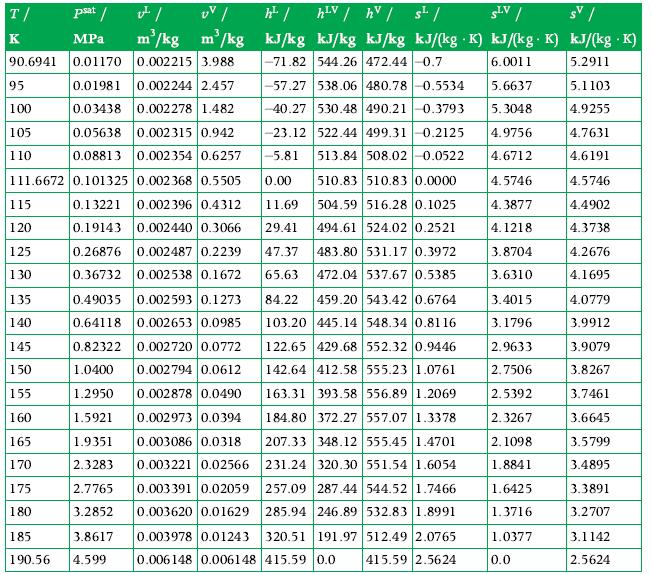
Data From Appendix A.5
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Thermodynamics Fundamentals And Engineering Applications
ISBN: 9780521862738
1st Edition
Authors: William C. Reynolds, Piero Colonna
Question Posted:





