Potassium, an alkali metal, enters a condenser in the vapor phase with a mass flow rate of
Question:
Potassium, an alkali metal, enters a condenser in the vapor phase with a mass flow rate of 0.02 kg/s and at a temperature and pressure of 1370 K and 0.4 MPa. It exits as a saturated liquid at 0.2 MPa. Calculate the rate of energy transfer as heat from the condenser in kW using Appendix A.11 for data.
Data From A.11
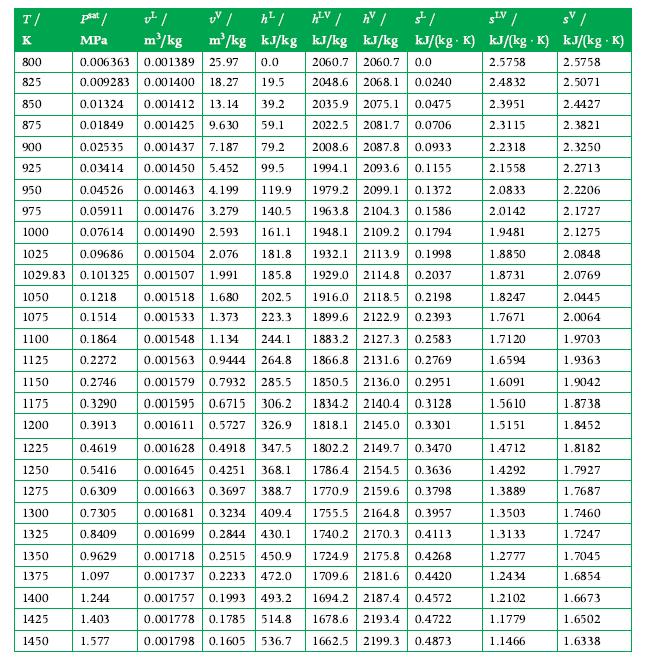
Transcribed Image Text:
T/ K 800 825 850 875 900 925 950 975 1000 1025 0.09686 1029.83 0.101325 0.1218 0.1514 0.1864 0.2272 0.006363 0.001389 25.97 0.0 0.009283 0.001400 18.27 19.5 0.01324 0.001412 13.14 39.2 2035.9 2075.1 0.0475 2022.5 2081.7 0.0706 0.02535 2008.6 2087.8 0.0933 0.01849 0.001425 9.630 59.1 0.001437 7.187 79.2 0.001450 5.452 99.5 1994.1 2093.6 0.1155 1979.2 2099.1 0.1372 1963.8 2104.3 0.1586 0.03414 0.04526 0.001463 4.199 119.9 0.05911 0.001476 3.279 140.5 161.1 0.07614 0.001490 2.593 0.001504 2.076 1948.1 2109.2 0.1794 1932.1 2113.9 0.1998 1929.0 2114.8 0.2037 0.001507 1.991 1050 0.001518 1.680 202.5 1916.0 2118.5 0.2198 1899.6 2122.9 0.2393 1075 0.001533 1.373 223.3 244.1 1100 0.001548 1.134 1883.2 2127.3 0.2583 1125 0.001563 0.9444 264.8 1866.8 2131.6 0.2769 1150 1175 1834.2 2140.4 0.3128 1818.1 2145.0 0.3301 1802.2 2149.7 0.3470 1786.4 2154.5 0.3636 1770.9 2159.6 0.3798 0.001579 0.7932 285.5 1850.5 2136.0 0.2951 0.001595 0.6715 306.2 1200 0.001611 0.5727 326.9 1225 0.4619 0.001628 0.4918 347.5 0.5416 0.001645 0.4251 368.1 0.6309 0.001663 0.3697 388.7 0.7305 0.001681 0.3234 409.4 0.8409 0.001699 0.2844 430.1 0.001718 0.2515 450.9 0.001737 0.2233 472.0 0.001757 0.1993 493.2 0.001778 0.1785 514.8 0.001798 0.1605 536.7 1755.5 2164.8 0.3957 0.9629 1.097 1740.2 2170.3 0.4113 1724.9 2175.8 0.4268 1709.6 2181.6 0.4420 1694.2 2187.4 0.4572 1678.6 2193.4 0.4722 1662.5 2199.3 0.4873 1.244 1.403 1.577 1250 1275 plat/ MPa 1300 1325 1350 1375 1400 1425 1450 v²/ h²/ m³/kg m³/kg kJ/kg 0.2746 0.3290 0.3913 HV/ hv/ है। SLV / sv / kJ/kg kJ/kg kJ/(kg. K) kJ/(kg K) kJ/(kg K) 2060.7 2060.7 0.0 2.5758 2.5758 2048.6 2.4832 2.5071 2.3951 2.4427 2.3115 2.3821 2.3250 2.2713 2.2206 2.1727 2.1275 2.0848 2.0769 181.8 185.8 2068.1 0.0240 2.2318 2.1558 2.0833 2.0142 1.9481 1.8850 1.8731 1.8247 1.7671 1.7120 1.6594 1.6091 1.5610 1.5151 1.4712 1.4292 1.3889 1.3503 1.3133 1.2777 1.2434 1.2102 1.1779 1.1466 2.0445 2.0064 1.9703 1.9363 1.9042 1.8738 1.8452 1.8182 1.7927 1.7687 1.7460 1.7247 1.7045 1.6854 1.6673 1.6502 1.6338
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
Mh 1 Neglecting kinetic energy at the inlet and outlet of the condenser the energy balance is Mh QMh ...View the full answer

Answered By

Krishnavendra Y
I am a self motivated financial professional knowledgeable in; preparation of financial reports, reconciling and managing accounts, maintaining cash flows, budgets, among other financial reports. I possess strong analytical skills with high attention to detail and accuracy. I am able to act quickly and effectively when dealing with challenging situations. I have the ability to form positive relationships with colleagues and I believe that team work is great key to performance. I always deliver quality, detailed, original (0% plagiarism), well-researched and critically analyzed papers.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Thermodynamics Fundamentals And Engineering Applications
ISBN: 9780521862738
1st Edition
Authors: William C. Reynolds, Piero Colonna
Question Posted:
Students also viewed these Engineering questions
-
The Stillwater geothermal power plant in Nevada, which started full commercial operation in 1986, is designed to operate with seven identical units. Each of these seven units consists of a pair of...
-
A power plant operates on a regenerative vapor power cycle with one closed feed water heater. Steam enters the first turbine stage at 10 MPa, 500oC and expands to 1 MPa, where some of the steam is...
-
HL Co. uses the high-low method to derive a total cost formula. Using a range of units produced from 1,500 to 7,500, and a range of total costs from $21,000 to $45,000, producing 2,000 units will...
-
Over the past 2 years, Jonas Cone has used a dollar-cost averaging formula to purchase $300 worth of FCI common stock each month. The price per share paid each month over the 2 years is given in the...
-
What is a keyword and how is it useful in searching the literature? Referencing your work
-
A company issued 8-year, 5% bonds with a par value of $350,000. The company received proceeds of $373,745. Interest is payable semiannually. The amount of premium amortized for the first semiannual...
-
You want to estimate the average SAT score for all students who took the Ethan-Davies SAT Preparation course during the past 2 years. You select a simple random sample of 100 such students from a...
-
also completed an additional 1 4 0 , 0 0 0 cookies. The department started but did not complete 4 , 0 0 0 in the month of October, which were 6 0 % complete. Direct materials are added at the...
-
Oxygen flows through an adiabatic steady flow compressor as saturated vapor at a rate of 1000 kg/hr. The saturated vapor enters at 2.5 bar and exits at 17.5 bar and 175 K. Calculate the shaft work...
-
An adiabatic pistoncylinder system contains a 1000 W immersion heater and 4 kg of water. The water is initially at 1 atm and 96% quality. The heater is operated for 7 minutes. Calculate the final...
-
Small business owner Jay Goltz described several decisions he made to reduce the fixed costs of his businesses, including replacing halogen lamps with LED lamps. Goltz noted, "I'm guessing that many...
-
The copper coil placed inside a stove with the purpose of heating water that flows through the coil. The coil is made from copper tube with an OD of 1 2 . 7 0 mm and ID of 1 1 . 0 8 mm . Water enters...
-
Confidence Levels Given specific sample data, such as the data given in Exercise 1, which confidence interval is wider: the 95% confidence interval or the 80% confidence interval? Why is it wider?
-
Yellow M&Ms Express the confidence interval (0.0847, 0.153) in the form of P - E < p < p + E. 12. Blue M&Ms Express the confidence interval 0.255 (+-) 0.046 in the form of P - E < p < p + E.
-
An ideal, noble gas with a mass of 97.2 g at 25 C and a pressure of 608 torr has a volume of 22.7 L. 1. What is the pressure (in atm)? SHOW ALL WORK. 2. What is R (number and units)? 3. What is the...
-
A drug is used to help prevent blood clots in certain patients. In clinical trials, among 4705 patients treated with the drug, 170 developed the adverse reaction of nausea. Construct a 95% confidence...
-
Can you think of any additional financial or banking services that banks could offer small-business owners?
-
What is a content filter? Where is it placed in the network to gain the best result for the organization?
-
The Fischer esterification reaction is given by, Determine the equilibrium constant K of this reaction in terms of the concentrations of the reactants c R-(C=O)-OH , c R-OH and of the products c...
-
Steel wool is placed inside a cylinder filled with molecular oxygen O 2 , considered as an ideal gas. A piston ensures a constant pressure of the gas. The steel wool reacts with the molecular oxygen...
-
Apply the general definition of the battery potential, to the Daniell cell ( 8.7.4) and show that it yields relation (8.108). Show that the battery potential can be written as, where - 1 zFF A VaA PA
-
What is the duration for the following bond with annual payments? 5.6300 5.7957 4.9894 5.1910 5.3806
-
DOLLAR TREE GROCERY OUTLET Short-Term Liquidity 2021 2022 2021 2022 Current Ratio 1.35 1.51 1.86 1.67 Quick Ratio 0.24 0.15 0.63 0.42 Cash Ratio Cash Conversion Cycle 34.78 45.75 19.41 21.61 Days...
-
A family has a $117,443, 25-year mortgage at 5.4% compounded monthly. (A) Find the monthly payment and the total interest paid. (B) Suppose the family decides to add an extra $100 to its mortgage...

Study smarter with the SolutionInn App


