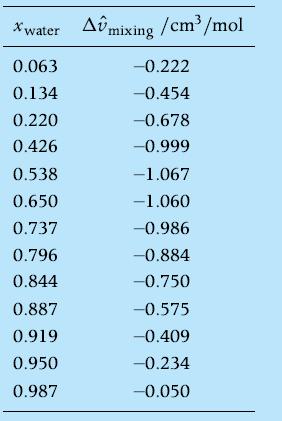
The following table reports the measurements of excess molar volume for the water ethanol mixture at T
Question:
The following table reports the measurements of excess molar volume for the water– ethanol mixture at T = 298 K of Figure 8.4.
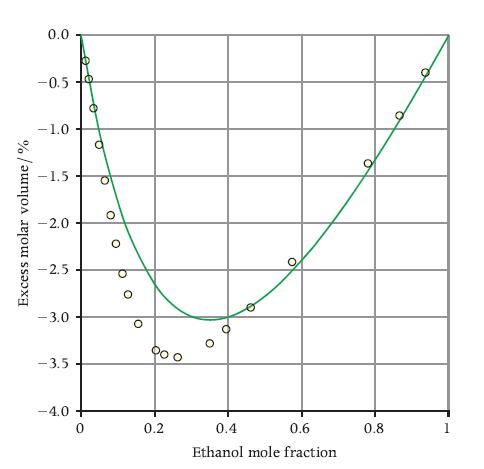
Data on excess molar properties are often correlated with a polynomial of the so-called Redlich–Kister form
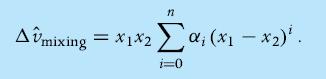
Correlate the given data with a Redlich–Kister polynomial of third degree, and thus calculate the coefficients of the polynomial.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Thermodynamics Fundamentals And Engineering Applications
ISBN: 9780521862738
1st Edition
Authors: William C. Reynolds, Piero Colonna
Question Posted:





