Water is cooled with ice cubes (Fig. 6.10). The water and the ice cubes are considered as
Question:
Water is cooled with ice cubes (Fig. 6.10). The water and the ice cubes are considered as an isolated system. Initially, the ice cubes are at melting temperature T0 and the water at temperature Ti. The total initial mass of ice is M' and the initial mass of water is M. The latent heat of melting of ice per unit mass is ![]() and the specific heat per unit mass of water is c*V.
and the specific heat per unit mass of water is c*V.
(a) Determine the final temperature Tm of the water.
b) Determine the final temperature Tm of the water if melted ice (i.e. water) had been added at melting temperature T0 instead of ice.
Numerical Application: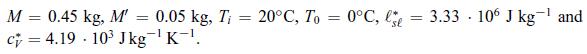
Figure 6.10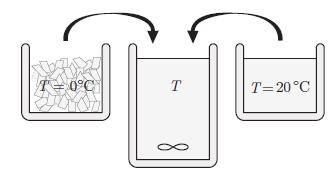
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Principles Of Thermodynamics
ISBN: 9781108426091
1st Edition
Authors: Jean-Philippe Ansermet, Sylvain D. Brechet
Question Posted:





