Careful measurements show that the specific heat of the solid phase depends on temperature (Fig. P17.117). How
Question:
(a) Will be shorter;
(b) Will be longer;
(c) Will be the same;
(d) Depends on the density of the cryoprotectant.
Figure P17.117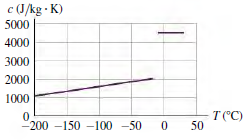
In cryopreservation, biological materials are cooled to a very low temperature to slow down chemical reactions that might damage the cells or tissues. It is important to prevent the materials from forming ice crystals during freezing. One method for preventing ice formation is to place the material in a protective solution called a cryoprotectant. Stated values of the thermal properties of one cryoprotectant are listed here:
Melting point .......................................................... -20€‘C
Latent heat of fusion .............................................. 2.80 × 105 J/kg
Specific heat (liquid) ............................................... 4.5 × 103 J/kg ˆ™ K
Specific heat (solid) ................................................. 2.0 × 103 J/kg ˆ™ K
Thermal conductivity (liquid) .................................... 1.2 W/m ˆ™ K
Thermal conductivity (solid) ..................................... 2.5 W/m ˆ™ K
Step by Step Answer:

University Physics with Modern Physics
ISBN: 978-0133977981
14th edition
Authors: Hugh D. Young, Roger A. Freedman





