Question: The binding energy per nucleon for most nuclides doesnt vary much (see Fig. 43.2). Is there similar consistency in the atomic energy of atoms, on
The binding energy per nucleon for most nuclides doesn€™t vary much (see Fig. 43.2). Is there similar consistency in the atomic energy of atoms, on an €œenergy per electron€ basis? If so, why? If not, why not?
Fig.43.2
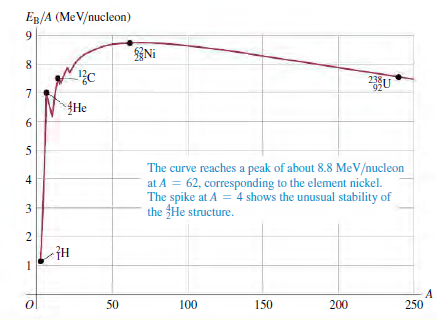
ER/A (MeV/nucleon) Ni 238U 92 {He The curve reaches a peak of about 8.8 MeV/nucleon at A = 62, corresponding to the element nickel. The spike at A = 4 shows the unusual stability of the He structure. 50 100 150 200 250 6. 4) 3.
Step by Step Solution
3.41 Rating (157 Votes )
There are 3 Steps involved in it
No As the number of electrons increases so does ... View full answer

Get step-by-step solutions from verified subject matter experts


