Question: Suggest reagents that could be used to prepare these alkyl halides from alcohols: CH3 CH, b) CH,CI ) C,r Ph CH;CH2 Br CI Br )
Suggest reagents that could be used to prepare these alkyl halides from alcohols:
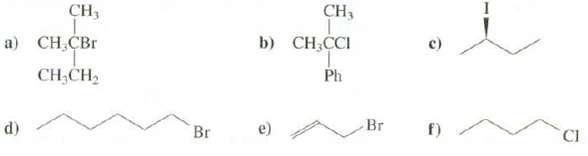
CH3 CH, b) CH,CI ) C,r Ph CH;CH2 Br CI Br ) d)
Step by Step Solution
3.45 Rating (164 Votes )
There are 3 Steps involved in it
CH3 a CH3COH CH3CH ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
15-C-O-E-R (54).docx
120 KBs Word File


