Suppose 0.825 mol of an ideal gas undergoes an isothermal expansion as energy is added to it
Question:
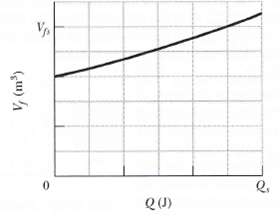
Suppose 0.825 mol of an ideal gas undergoes an isothermal expansion as energy is added to it as heat Q. It Figure 19-20 shows the final volume V1versus Q, what is the gas temperature? The scale of the vertical axis is set by Vfs = 0.30 m3, and the scale of the horizontal axis is set by Qs =1200J.
Transcribed Image Text:
Q()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
Using Eq 1914 we note that since it is an isothermal process invol...View the full answer

Answered By

Mary Njunu
I posses Vast, diversified knowledge and excellent grammar as a result of working in ACADEMIC WRITING for more than 5 years. I deliver work in various disciplines with assurance of quality work. I purpose at meeting the clients’ expectations precisely. Let’s work together for the best and phenomenal grades.
4.90+
929+ Reviews
2557+ Question Solved
Related Book For 

Fundamentals of Physics
ISBN: 978-0471758013
8th Extended edition
Authors: Jearl Walker, Halliday Resnick
Question Posted:
Students also viewed these Thermodynamics questions
-
A quantity of an ideal gas undergoes an isothermal expansion at 20 oC and does 3.0 x 103 J of work on its surroundings in the process. (a) Will the entropy of the gas (1) increase, (2) remain the...
-
Suppose 4.00 mol of an ideal gas undergoes a reversible isothermal expansion from volume V1 to volume V2 = 2.00 V1 at temperature T = 400 K. Find (a) The work done by the gas and (b) The entropy...
-
The temperature of 0.150 mol of an ideal gas is held constant at 77.0oC while its volume is reduced to 25.0% of its initial volume. The initial pressure of the gas is 1.25 atm. (a) Determine the work...
-
The following MINITAB output exhibits the cumulative distribution function curves of three lognormal distributions. Their mean and variance, respectively, are (1, .5), (1, 1), and (1, 2). Compare the...
-
In 2014, John opened an investment account with Randy Hansen, who held himself out to the public as an investment adviser and securities broker. John contributed $200,000 to the account in 2014. John...
-
Fincher Manufacturing has projected sales of $135 million next year. Costs are expected to be $76 million and net investment is expected to be $15 million. Each of these values is expected to grow at...
-
CALCULATING AMOUNT A AILABLE AT RETIREMENT. Jocelyn Booker, a 25-year-old personal loan of- ficer at Second National Bank, understands the importance of starting early when it comes to saving for...
-
On October 15, 1990, United Airlines (UAL Corporation) placed the largest wide-body aircraft order in commercial aviation history60 Boeing 747-400s and 68 Boeing 777swith an estimated value of $22...
-
Question 1 options: Financial data for Beaker Company for last year appear below: Beaker Company Statements of Financial Position Beginning Balance Ending Balance Assets: Cash $ 290,000 $ 451,280...
-
Complete an SFAS Matrix and a TOWS Matrix on your Strategic Audit firm using your EFAS Start with your EFAS and IFAS assignments. O Make all corrections to these assignments. The SFAS matrix is to...
-
Water bottle in a hot car in the American Southwest, the temperature in a closed car parked in sunlight during the summer can be high enough to burn flesh. Suppose a bottle of water at a refrigerator...
-
In the temperature range 310 K to 330 K, the pressure p of a certain non ideal gas is related to volume V and temperature T by how much work is done by the gas if its temperature is raised from 315 K...
-
Batteries and fuel cells employ a. oxidation reactions only b. reduction reactions only c. both oxidation and reduction reactions d. acid-base neutralization reactions
-
Consider the following account balances (in thousands) for the Shaker Corporation In the Dec 31.2021 Cash $200,000 and Capital $2,000,000 and Retained earnings $1,500,000 The balances of raw...
-
Unless otherwise stated, assume gravitational acceleration g = 9.81 m/s and the density of water to be 1000 kg/m. Unless otherwise stated, give all numerical answers to 3 significant figures, such as...
-
The purpose of this installment is to classify stock, bond, and mutual fund investments, explore tools for their evaluation and select these securities based on your investment philosophy and goals....
-
Jackson County Senior Services is a nonprofit organization devoted to providing essential services to seniors who live in their own homes within the Jackson County area. Three services are provided...
-
Caldwell (2003) explores differences between the roles of leaders and managers. "Leaders...envision, initiate, or sponsor strategic change of a far-reaching or transformational nature. In contrast,...
-
Describe the four steps in the CBR process.
-
During the month, services performed for customers on account amounted to $7,500 and collections from customers in payment of their accounts totaled $6,000. At the end of the month, the Accounts...
-
If you deposit $2,000 in a 5-year certificate of deposit at 5.2%, how much will it be worth in five years?
-
At the surface of Jupiter's moon 10, the acceleration due to gravity is g = 1.81 mfs2. A watermelon weighs 44.0 N at the surface of the earth. (a) What is the watermelon's mass on the earth's...
-
An astronaut's pack weighs 17.5 N when she is on earth but only 3.24 N when she is at the surface of an asteroid. (a) What is the acceleration due to gravity on this asteroid? (b) What is the mass of...
-
World-class sprinters can accelerate out of the starting blocks with an acceleration that is nearly horizontal and has magnitude 15 m/s2. How much horizontal force must a 55-kg sprinter exert on the...
-
question 1- You borrow a simple loan of SR 500,000, interest rate is 20%, it matures in one year. what's the yied to maturity? question 2- calculate_i for One-Year Discount Bond with price(p) =...
-
Taste of Muscat is a reputed chain of restaurants operating in Oman. Assume You are working as a management accountant for this restaurant chain which is specialized in all types of Arabic food. Your...
-
Industry Current Year Minus 1 Current Year Minus 2 Company: Air Products and Chemicals, Inc. (APD) Stock Price: 306.72 USD Shares Outstanding: 220.89 M Financial Ratios Most Current Year Current...

Study smarter with the SolutionInn App


