Tamoxifen is a drug used in the treatment of breast cancer, how would you prepare tamoxifen from
Question:
Tamoxifen is a drug used in the treatment of breast cancer, how would you prepare tamoxifen from benzene, the following ketone, and any other reagents needed?
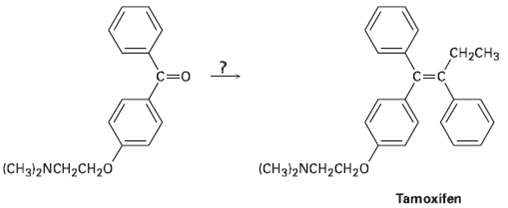
Transcribed Image Text:
CH2CH3 C=0 C=c (CH3!2NCH2CH20 (CH3)2NCH2CH20 Tamoxifen
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 52% (19 reviews)
i CH3CHCCI AICI3 1 Br FeBr3 2 Mg ether OH CHCHCH3 PBr3 CCHCH3 MgBr Br 1 LIAIH4 ...View the full answer

Answered By

Moses mwangi
With prior writing experience, be sure that I will give a great grade, If not an A+, it will be something close to this. My reviews speaks it all, Try me!!
4.80+
78+ Reviews
157+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Tamoxifen is a drug often used to treat breast cancer patients. One effect of the drug is to change the levels of cortisol-binding globulin (CBG). One study attempted to see if the effect of...
-
How would you prepare o-hydroxyphenyl-acetaldehyde from phenol? More than one step is required. HO o-Hydroxyphenylacetaldehyde CH-CO
-
How would you prepare pentanal from the following starting materials? (a) CH 3 CH 2 CH 2 CH 2 CH 2 OH (b) CH 3 CH 2 CH 2 CH 2 CH = CH 2 (c) CH 3 CH 2 CH 2 CH 2 CO 2 CH 3
-
Use a linear interpolation to estimate properties of ammonia to fill out the table below P [kPa] T [ C] v [m3/kg] x a) 550 0.75 b) 80 20 c) 10 0.4
-
1. Do you think the model used by Seats2Meet would work in the United States? Why or why not? 2. In what ways can social capital help you in running a start-up firm or doing freelance projects?
-
For each of the following, state whether F = 0 for a main effect, the A B interaction, or both. a. Cell means are equal. b. Row totals are equal. c. Column totals are equal.
-
Suppose a new and much more liberal Congress and administration were elected, and their first order of business was to take away the independence of the Federal Reserve System, and to force the Fed...
-
Down Home Jeans Co. has an annual plant capacity of 65,000 units, and current production is 45,000 units. Monthly fixed costs are $40,000, and variable costs are $22 per unit. The present selling...
-
Explain the answers in details... 1 - Suppose that f ( 0 , 1 , 2 ) = 2 % and r ( 0 , 2 ) = 3 % Which of the following is / are true? Select one or more alternatives: A - r ( 0 , 1 ) = 3 % B - The...
-
Neveranerror Inc. was organized on June 2, 2010, by a group of accountants to provide accounting and tax services to small businesses. The following transactions occurred during the first month of...
-
When Cyclohexanone is heated in the presence of a large amount of acetone cyanohydrin and a small amount of base, Cyclohexanone cyanohydrin and acetone arc formed. Propose amechanism. HO CN OH "OH +...
-
Paraldehyde, a sedative and hypnotic agent, is prepared by treatment of acetaldehyde with an acidic catalyst. Propose a mechanism for the reaction. . C H+ H catalyst C Paraldehyde
-
With reference to Exercise 2.14 on page 34, draw a boxplot. Data From Exercise 2.14 2.14 An engineer uses a thermocouple to monitor the tem- perature of a stable reaction. The ordered values of 50...
-
3 Below is financial information for December Inc., which manufactures a single product: 4 5 5 7 3 #units produced October Low activity November High activity 7,000 11,000 Cost of goods manufactured...
-
(b) The satellite's booster rockets fire and lift the satellite to a higher circular orbit of radius 2R1. The satellite follows the path shown in the diagram below, moving a total distance S during...
-
D. An airplane flies at a speed of 250 kilometers per hour (kph) at an altitude of 3000 m. Assume the transition from laminar to turbulent boundary layers occurs at critical Reynolds Number, RE cr, =...
-
1. (35 points) by Qet = QoQ = Q0Q2 N!37 N/A (1-B, (r), where A- B(T) V * 4R (e-(R)/KT - 1) R dR is the second virial coefficient. The classical partition function for an imperfect gas comprising N...
-
es Farm has 28 employees who are paid biweekly. The payroll register showed the following payroll deductions for the pay period ending March 23, 2021. Gross Pay 85,950.00 EI Premium Income Taxes...
-
During an assessment of the risk associated with sales contracts and related commissions, which of the following factors would most likely result in an expansion of the engagement scope? (a) An...
-
The Adjusted Trial Balance columns of a 10-column work sheet for Webber Co. follow. Complete the work sheet by extending the account balances into the appropriate financial statement columns and by...
-
Use the VSEPR theory to predict the shapes of the anions (a) ClO 4 - ; (b) S 2 O 3 2- (that is, SSO 3 2- ); (c) PF 6 - ; (d) I 3 - .
-
The acid-catalyzed aldol condensation of acetone (just shown) also produces some 2, 6-dimethylhepta-2, 5-dien-4-one. Give a mechanism that explains the formation of this product.
-
Heating acetone with sulfuric acid leads to the formation of mesitylene (1, 3, 5-trimethylbenzene). Propose a mechanism for this reaction.
-
(a) Provide a mechanism for the aldol addition of propanal shown here. (b) How can you account for the fact that the product of the aldol addition is 3-hydroxy- 2-methylpentanal and not...
-
Which of the following accounts will not be closed during the closing process? a. Accounts Recelvable b. Wages Expense c. Fees Earned d. Rent Expense
-
Clarkson Lumber Company After a rapid growth in its business during recent years, the Clarkson Lumber Company, in the spring of 1996, anticipated a further substantial increase in sales. Despite good...
-
How do external factors such as changing consumer preferences affect the retail industry?"

Study smarter with the SolutionInn App


