Tetracaine is a substance used medicinally as a spinal anesthetic during lumbar punctures (spinal taps). (a) How
Question:
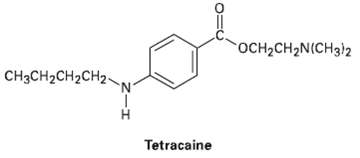
Tetracaine is a substance used medicinally as a spinal anesthetic during lumbar punctures (spinal taps).
(a) How would you prepare tetracaine from the corresponding aniline derivative, ArNH2?
(b) How would you prepare tetracaine from p-nitro benzoic acid?
(c) How would you prepare tetracaine frombenzene?
Transcribed Image Text:
OCH2CH2N(CH3)2 CHяCH2CH2CH2- Tetracaine
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 45% (11 reviews)
CH3CH23NH 0N b 0N Tetracaine COCHCHNCH32 H Pt NCH EtOH COCHCHNCH32 COH ...View the full answer

Answered By

Antony Mutonga
I am a professional educator and writer with exceptional skills in assisting bloggers and other specializations that necessitate a fantastic writer. One of the most significant parts of being the best is that I have provided excellent service to a large number of clients. With my exceptional abilities, I have amassed a large number of references, allowing me to continue working as a respected and admired writer. As a skilled content writer, I am also a reputable IT writer with the necessary talents to turn papers into exceptional results.
4.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How would you prepare aniline from the following starting materials? (a) Benzene (b) Benzamide (c) Toluene
-
How would you prepare o-hydroxyphenyl-acetaldehyde from phenol? More than one step is required. HO o-Hydroxyphenylacetaldehyde CH-CO
-
How would you prepare 72.5 g of an aqueous solution that is 5.00% potassium iodide, KI, by mass?
-
Coin Flips You flip a coin 100 times and get 58 heads and 42 tails. Calculate the chi-square statistic by hand showing your work, assuming the coin is fair.
-
Use one of the websites listed in Table 13.1 on page 357 to find a job opening in your target profession. If you haven't narrowed down to one career field yet, chose a business job for which you will...
-
Laboratory scientists have created the electric and magnetic fields shown in FIGURE EX31.3. These fields are also seen by scientists who zoom past in a rocket traveling in the x-direction at 1.0 10...
-
What, if any, impact do you think the results of this bargaining process will have when the agreement is next negotiated?
-
The firm of Bell & Greer, CPAs, has been asked to perform attest services for Trek Corporation (a nonpublic company) for the year ended December 31, Year 5. Bell & Greer has two offices: one in Los...
-
1 You will receive $1,000 at the end of each year for the next five years. How much would you accept now in exchange for your right to receive $1,000 at the end of each year for the next five years?...
-
The Purple Company This project will give you an opportunity to apply your knowledge of accounting principles and procedures to a corporation. You will handle the accounting work of The Purple...
-
Cyclopentamine is an amphetamine-like central nervous system stimulant. Propose a synthesis of Cyclopentamine from materials of five carbons orless. CH3 -CH2CHNHCH3 Cyclopentamine
-
Atropine, C 17 H 23 NO 3 , is a poisonous alkaloid isolated from the leaves and roots of Atropa belladonna, the deadly nightshade. In small doses, atropine acts as a muscle relaxant; 0.5ng (nanogram,...
-
Discuss the changing role of women throughout the eighteenth century.
-
If a change were made to Technical Spec 2 in the product's design, this would likely change the customer's opinion of which value feature the most? Quick Start Quick Start QFD Matrix 1 = Strong...
-
You are a quality management consultant for the Beserk Tennis Ball Company. Beserk is redesigning its current model of tennis ball, and you are asked to use QFD analysis to make suggestions about...
-
You are reviewing a tender evaluation that is to be awarded on lowest total price. The bid evaluations follow: To which company should the contract be awarded? Company Capital Cost Maintenance...
-
You have invited four companies to bid on a consulting project. All four companies answered your invitation to tender, but the bids vary in the number of hours each company estimates will be required...
-
Boston Cycles inventory data for the year ended December 31, 2011, follow: Assume that the ending inventory was accidentally overstated by $2,200. Requirement 1. What are the correct amounts for cost...
-
Identify the steps taken in the union organization process.
-
Evaluate the line integral, where C is the given curve. C x 2 dx + y 2 dy, C consists of the arc of the circle x 2 + y 2 = 4 from (2, 0) to (0, 2) followed by the line segment from (0, 2) to (4, 3)
-
Which factors affect the relative acidity of an oxyacid?
-
Which of the following compounds can exist as cis-trans isomers? If such isomerism is possible, draw the structures in a way that clearly illustrates the geometry. a. 3-octene b. 3-chloropropene c....
-
The mold metabolite and antibiotic mycomycin has the formula: Number the carbon chain, starting with the carbonyl carbon. a. Which multiple bonds are conjugated? b. Which multiple bonds are...
-
Write structural formulas for the following: a. 1,4-dichloro-2-pentene b. 3-hexyne c. 1,2-diethylcyclobutene d. 2-bromo-1,3-pentadiene
-
September 1 . Purchased a new truck for $ 8 3 , 0 0 0 , paying cash. September 4 . Sold the truck purchased January 9 , Year 2 , for $ 5 3 , 6 0 0 . ( Record depreciation to date for Year 3 for the...
-
Find the NPV for the following project if the firm's WACC is 8%. Make sure to include the negative in your answer if you calculate a negative. it DOES matter for NPV answers
-
What is the value of a 10-year, $1,000 par value bond with a 12% annual coupon if its required return is 11%?

Study smarter with the SolutionInn App


