The 1H NMR spectrum shown is that of an ether with the formula C4H8O. Propose astructure. TMS
Question:
The 1H NMR spectrum shown is that of an ether with the formula C4H8O. Propose astructure.
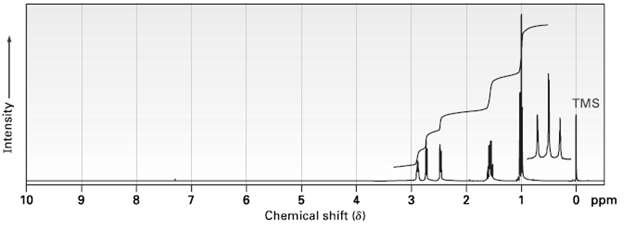
Transcribed Image Text:
TMS 10 9. 8. 7. 6. O ppm Chemical shift (8) Intensity 3.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 43% (16 reviews)
CH3CHCCH H 12Epox...View the full answer

Answered By

Rohith Bellamkonda
I am studying in IIT Indore,the most prestigious institute of India.I love solving maths and enjoy coding
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The 1H NMR spectrum shown is that of 3-methyl-3-buten-l-ol. Assign all the observed resonance peaks to specific protons, and account for the splittingpatterns. TMS H H2H20 10 8. O ppm Chemical shift...
-
The 1 H NMR spectrum shown is that of a compound with formula C 9 H 10 O. How many double bonds and/or rings does this compound contain? If the unknown has an IR absorption at 1690 cm ?1 , what is a...
-
The 1 H NMR spectrum shown is that of a compound isomeric with the one in Problem 19.65. This isomer has an IR absorption at 1730 cm ?1 . Propose a structure. TMS 10 O ppm 1. Chemical shift (8)...
-
Provide a brief description of the seven basic steps to use JDBC.
-
Would you feel the same way about having biometric information on your driver's license as on your passport? Why or why not?
-
The government website fuel economy. gov has data on thousands of cars regarding their fuel efficiency. A random sample from this website of SUVs manufactured between 2012 and 2015 gives the...
-
2. In the years subsequent to the sale of plant assets, why would the investment and/or noncontrolling interest accounts be used to adjust the unrealized gains or losses from the sale of plant assets?
-
Wicker Works Inc.'s budgeted unit sales for the year 2013 were: Tables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30,000 Chairs . . . . . . . . . . . ....
-
Cost of Goods Manufactured and sold The following data relate to three independent production periods of Riverside Manufacturing Company. Missing data are indicated by question marks. A B C...
-
For hierarchical routing with 4800 routers, what region and cluster sizes should be chosen to minimize the size of the routing table for a three-layer hierarchy? A good starting place is the...
-
2-Butene-1-thiol is one component of shunk spray. How would you synthesize this substance from methyl 2-butenoate? From 1,3-butadiene CH3CH=CHCOCH3 CH3CH=CHCH2SH 2-Butene-1-thiol Methyl 2-butenoate
-
Give IUPAC name for the following compounds (reddish brown =Br): (a) (b) (c)
-
Mega Mart has two employees in 2010. Marsha earns \(\$ 4,200\) per month and Tom, the manager, earns \(\$ 10,000\) per month. Neither is paid extra if they work overtime. Assume the Social Security...
-
Which industries gain and which industries lose from the availability of cheap natural gas produced from shale deposits? Joseph Schumpeter, an Austrian-born economist who emigrated to the United...
-
Did the value of the Canadian dollar rise or fall between Tuesday and Wednesday?
-
As vice president for community relations, you want to explore the possibility of developing service learning programs with several nearby colleges and universities. Using Figure 2.5, suggest the...
-
Your organization initiated a project to raise money for an important charity. Assume that there are 1,000 people in your organization. Also, assume that you have six months to raise as much money as...
-
A \(20-\mathrm{cm}\)-long rod, with uniform linear charge density \(100 \mathrm{nC} / \mathrm{cm}\), is set up symmetrically on the \(x\) axis. What are the magnitude and direction of the electric...
-
To understand the procedure for verifying the work eligibility of potential employees before offering them employment. AppendixLO1
-
Listed below are common types of current liabilities, contingencies, and commitments: a. Accounts payable b. Bank loans and commercial paper c. Notes payable d. Dividends payable e. Sales and excise...
-
One kilogram of saturated liquid methane at 160 K is placed in an adiabatic pistion-and-cylinder device, and the piston will be moved slowly and reversibly until 25 percent of the liquid has...
-
Trans-1-Phenyl-1, 3-butadiene has max = 280 ( = 27,000) calculate the concentration of a solution that has A = 0.643 at 280nm in a 1 cm cell.
-
Nitro methane max = 275nm ( = 1.5) what kind of transition is responsible for this absorption?
-
3-Buten-2-one has max =213nm ( = 7080) and max = 320nm ( = 21) what kind of transition is responsible for each of these absorptions?
-
Compute the value of ordinary bonds under the following circumstances assuming that the coupon rate is 0.06:(either the correct formula(s) or the correct key strokes must be shown here to receive...
-
A tax-exempt municipal bond has a yield to maturity of 3.92%. An investor, who has a marginal tax rate of 40.00%, would prefer and an otherwise identical taxable corporate bond if it had a yield to...
-
Please note, kindly no handwriting. Q. Suppose a 3 year bond with a 6% coupon rate that was purchased for $760 and had a promised yield of 8%. Suppose that interest rates increased and the price of...

Study smarter with the SolutionInn App


