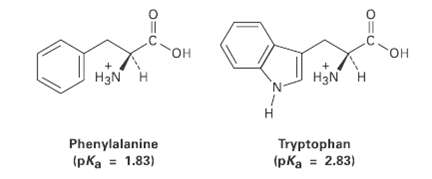
The amino acid phenylalanine has pKa = 1.83, and tryptophan has pKa = 2.83. Which is the
Question:
The amino acid phenylalanine has pKa = 1.83, and tryptophan has pKa = 2.83. Which is the strongeracid?
Transcribed Image Text:
он он OH H3N H H3N H н Tryptophan Phenylalanine (pka = 1.83) (pka = 2.83)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (10 reviews)
Recall from Section 28 that a stro...View the full answer

Answered By

Rashul Chutani
I have been associated with the area of Computer Science for long. At my university, I have taught students various Computer Science Courses like Data Structures, Algorithms, Theory of Computation, Digital Logic, System Design, and Machine Learning. I also write answers to questions posted by students in the area of and around Computer Science.
I am highly fortunate to receive great feedback on my teaching skills that keeps me motivated. Once a student sent me an email stating that I had explained to him a concept better than his professor did.
I believe in the fact that "Teaching is the best way to learn". I am highly fascinated by the way technology nowadays is solving real-world problems and try to contribute my bit to the same.
Besides tutoring, I am a researcher at the Indian Institute of Technology. My present works are in the area of Text Summarization and Signal and Systems.
Some of my achievements include clearing JEE Advanced with an All India Rank of 306 out of 1.5 million contesting candidates and being the Department Ranker 1 at my University in the Department of Computer Science and Engineering.
I look forward to providing the best Tutoring Experience I can, to the student I teach.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Acid HA has pKa = 20; acid HB has pKa = 10. (a) Which is the stronger acid? (b) Will an acid-base reaction with an equilibrium lying to the right take place if Na+A- s added to HB? Explain your...
-
The acid HA has pKa = 7.00. (a) Which is the principal species, HA or A-, at pH 6.00? (b) Which is the principal species at pH 8.00? (c) What is the quotient [A-]/[HA] at pH 7.00? at pH 6.00?
-
The amino acid cysteine has the structure shown: (a) A second sulfur-containing amino acid called cystine (C6H12N2O4S2) is formed when cysteine undergoes biological oxidation. Suggest a reasonable...
-
Why is it so difficult to assess whether a firm is properly valued?
-
Cisco, a calendar year taxpayer who is age 63, owns a residence in which he has lived for 21 years. The residence is destroyed by fire on August 8, 2016. The adjusted basis is $190,000, and the fair...
-
1. Why would a search company such as Google decided to enter the fiber services business? Describe the benefits that Google expects to obtain from this new business venture. 2. Describe the various...
-
building another probability distribution, this time a distribution of mean differences
-
Kawmin is a small country that produces and consumes jelly beans. The world price of jelly beans is $1 per bag, and Kawmins domestic demand and supply for jelly beans are governed by the following...
-
1-magine you work in the economic development department for a small town or municipality. You have been tasked to write a briefing note to the council on how to adopt CED strategies into their...
-
Construct a short-term financial plan for Springfield Snowboards based on its expansion opportunity described in the "Positive Cash Flow Shocks" part of Section 20.1. Base the plan on the following...
-
Nitric acid (HNO3) reacts with ammonia (NH3) to yield ammonium nitrate. Write the reaction, and identify the acid, the base, the conjugate acid product, and the conjugate base product.
-
Amide ion, H 2 N , is a stronger base then hydroxide ion, HO . Which is the stronger acid, NH 3 or H 2 O? Explain.
-
Was BlackBerry smart to refocus its position toward security-related technologies? Should BlackBerry still be competing in the smartphone industry?
-
Please help. I would really appreciate it. Question 1. Polly owns an electric power plant in the city of Newtown. The market price of electricity in Newtown is $1.00 per kilowatt hour (kwh). Polly's...
-
(Appendix 3A) Jenson Manufacturing is developing cost formula for future planning and cost control. Utilities is one of the mixed costs associated with production. The cost analyst has suggested that...
-
A company has the following trial balance as at 31 December 2015: TRIAL BALANCE AS AT 31 DEC 2015 Dr Cr Sales Revenue 125 000 Purchases 78 000 Carriage 4 000 Electricity and rent 5 100 Administrative...
-
What gets printed to the screen by the following segment of code? String str1 = "hello"; String str2 = "world"; if (!strl.equals(str2)) { System.out.println(str1+" "+str2); } else { }...
-
a) Consider the following financial data (in millions of dollars) for Costello Laboratories over the period of 2014-2018: Year Sales Net income Total assets Common equity 2014 $3,800 $500 $3,900...
-
Which statement is least appropriate? The IS auditors role in disaster planning is to: a. recommend that a plan is in place b. independently test this plan c. call out key contacts in the event of an...
-
List four items of financial information you consider to be important to a manager of a business that has been operating for a year.
-
a. Using only the steam tables, compute the fugacity of steam at 400C and 2 MPa, and at 400C and 50 MPa. b. Compute the fugacity of steam at 400C and 2 MPa using the principle of corresponding...
-
Name the following compounds: a) c) H C CH3 b) d)
-
Draw structures for these compounds: (a) 1, 1-Dimethylcylohexane (b) Ethylcyclopropane
-
Name these compounds: 22 b)
-
nformation pertaining to Noskey Corporation s sales revenue follows: November 2 0 2 1 ( Actual ) December 2 0 2 1 ( Budgeted ) January 2 0 2 2 ( Budgeted ) Cash sales $ 1 0 5 , 0 0 0 $ 1 1 5 , 0 0 0...
-
The management team of Netflix maintains a stable dividend using the Lintner model: Dt+1 = Dt + EPS Target Payout Where Dt (Dt+1) = dividend in the current period t (the next period t + 1) EPSt =...
-
#1 #2 hapter 50 10 D Werences lav Help Required information [The following information applies to the questions displayed below) Archer Company is a wholesaler of custom-built air-conditioning units...

Study smarter with the SolutionInn App


