Question: The diffusion coefficients for iron in nickel are given at two temperatures: (a) Determine the values of D 0 and the activation energy Q d
The diffusion coefficients for iron in nickel are given at two temperatures:
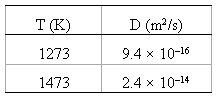
(a) Determine the values of D0 and the activation energy Qd.
(b) What is the magnitude of D at 1100ºC (1373 K)?
D (m?/s) T (K) 9.4 x 10-16 1273 1473 2.4 x 10-14
Step by Step Solution
3.44 Rating (180 Votes )
There are 3 Steps involved in it
a Using Equation 59a we set up two simultaneous equations with Q d and D 0 as unknowns as follows No... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
33-E-M-S-E-M-S (146).docx
120 KBs Word File


