The equilibrium separation of the K+ and Cl- ions in KCl is about 0.267 nm. (a) Calculate
Question:
The equilibrium separation of the K+ and Cl- ions in KCl is about 0.267 nm.
(a) Calculate the potential energy of attraction of the ions assuming them to be point charges at this separation.
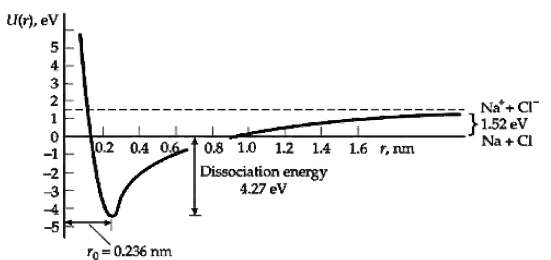
(b) The ionization energy of potassium is 4.34 eV and the electron affinity of Cl is 3.62 eV. Find the dissociation energy neglecting any energy of repulsion. (See Figure) The measured dissociation energy is 4.49 eV. What is the energy due to repulsion of the ions at the equilibriumseparation?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals of Ethics for Scientists and Engineers
ISBN: 978-0195134889
1st Edition
Authors: Edmund G. Seebauer, Robert L. Barry
Question Posted:





