The following model is that of an allylic carbocation intermediate formed by Protonation of a conjugated diene
Question:
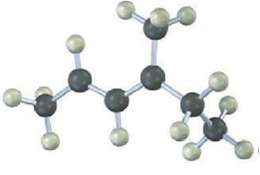
The following model is that of an allylic carbocation intermediate formed by Protonation of a conjugated diene with HBr. Show the structure of the diene and the structures of the final reactionproducts.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 61% (13 reviews)
HC H HC CH3 HC OH H Br C H CH3 or CH3 HC CHCH3 B...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The following model is that of an aldohexose: (a) Draw Fischer projections of the sugar, its enantiomer, and a diastereomer. (b) Is this a D sugar or an L sugar? Explain. (c) Draw the ? anomer of the...
-
The following model is specified:y 1 = 1 y 2 + 11 x 1 + 1 ,y 2 = 2 y 1 + 22 x 2 + 32 x 3 + 2 . All variables are measured as deviations from their means. The sample of 25 observations produces...
-
The following model is specified: y1 = 1y2 + 11x1 + 1, y2 = 2y1 + 22x2 + 32x3 + 2. All variables are measured as deviations from their means. The sample of 25 observations produces the following...
-
The following Excel output summarizes the results of an analysis of variance experiment in which the treatments were three different hybrid cars and the variable measured was the miles per gallon...
-
Suppose you work in the HR function at Nissan when it is identifying employees to work on the joint manufacturing project in Mexico. Briefly advise the company on how to prepare these employees to...
-
Solve the linear systems in the given exercises. Data From Exercise 28 , %3D1 2x, + 3x, + x, = 0 X X1 2X3 , + 2x, 2, 3 0
-
Know the difference between rational, bounded rational, and intuitive decisions.
-
Condensed financial data of Minnie Hooper Company are shown below. Additional information:1. New plant assets costing $146,000 were purchased for cash during the year.2. Investments were sold at...
-
The following T-account is a summary of the Cash account of Bonita Company. Cash (Summary Form) Balance, Jan. 1 9,000 Receipts from customers 365,600 Payments for goods 252.800 Dividends on stock...
-
Exhibit 4.22 presents selected operating data for three retailers for a recent year. Macy??s operates several department store chains selling consumer products such as brand-name clothing, china,...
-
The following diene does not undergo Diels?Alder reactions. Explain.
-
Give IUPAC names for the followingcompounds: (b) HCHHCHCHCH3 (a) CCHH CH (e) CCHHH3 (d) CH2CH2CH3 CCH3CCH3C2
-
What effect has NAFTA had on Canadian Trade?
-
Section Three Answer the questions below 1.While pulling out of her driveway, Bethany becomes distracted by a bee and strikes Melanie, who is riding past on a bicycle. Bethany suffers serious injury...
-
A __________ is a schedule periodic check of a specific process behavior. Question 1Answer A. Widget B. Dashboard C. Monitor D. Process ID
-
1. Was VAAF contractually obligated to pay Chad for refraining from smoking? 2. Was there consideration to support its promise to pay $500? 3. Are there other facts you need to know to make that...
-
Presented here are the comparative balance sheets of Hames Incorporated at December 31, 2023 and 2022. Sales for the year ended December 31, 2023, totaled $1,700,000.%0D%0A%0D%0AHAMES...
-
McDonald's conducts operations worldwide and is managed in two primary geographic segments: US, and International Operated Markets, which is comprised of Australia, Canada, France, Germany, Italy,...
-
What is the value of measures of central tendency and dispersion?
-
The following data are supplied for the common stocks of Nikola Corporation, Tesla, Inc. and General Motors: Nikola Corp (NKLA) Tesla Inc. (TSLA) Close Price ($) Close Price ($) 67.53 30.00 40.81...
-
A solution is an equimolar mixture of two volatile components A and B. Pure A has a vapor pressure of 50 torr, and pure B has a vapor pressure of 100 torr. The vapor pressure of the mixture is 85...
-
Predict the major products of the following reactions. (a) toluene + excess Cl2 (heat, pressure) (b) benzamide (PhCONH2) + Na (;oqioed NH3, CH3CH2OH) (d) o-xylene + H2 (1000 psi, 100oC, Rh catalyst)...
-
Predict the major products of treating the following compounds with hot, concentrated potassium permanganate, followed by acidification with dilute HCl. (a) isopropylbenzene (b) p-xylene (c) tetralin)
-
Propose a mechanism for the bromination of ethylbenzene shown above.
-
Duncan Inc. issued 500, $1,200, 8%, 25 year bonds on January 1, 2020, at 102. Interest is payable on January 1. Duncan uses straight-line amortization for bond discounts or premiums. INSTRUCTIONS:...
-
WISE-HOLLAND CORPORATION On June 15, 2013, Marianne Wise and Dory Holland came to your office for an initial meeting. The primary purpose of the meeting was to discuss Wise-Holland Corporation's tax...
-
Stock in ABC has a beta of 0.9. The market risk premium is 8%, and T-bills are currently yielding 5%. The company's most recent dividend is $1.60 per share, and dividends are expected to grow at a 6%...

Study smarter with the SolutionInn App


