The glycolysis pathway shown in figure has a number of intermediates that contain phosphate groups. Why can
Question:
The glycolysis pathway shown in figure has a number of intermediates that contain phosphate groups. Why can 3-phosphoglyceryl phosphate and phosphoenolpyruvate transfer a phosphate group to ADP while glucose 6-phosphatecannot?
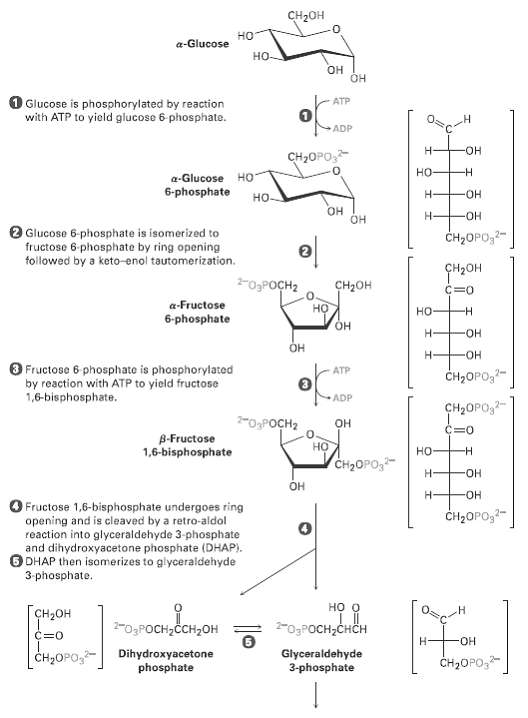
Transcribed Image Text:
CH2он но a-Glucose но- он OH O Glucose is phosphorylated by reaction with ATP to yield glucose 6 phosphate. ATP ADP н- HO- сн-OPО, но a-Glucose HO 6-phosphate H- HO- но- Он он -HO- Glucose 6-phosphate is isomerized to fructose 6 phosphate by ring opening tollowed by a keto-enol tautomerization. CH2OPO,2- ҫнрон -O3POCH2 CH2он C=0 a-Fructose но но 6-phosphate Н HO- Он ОН O Fructose 6 phosphate is phosphorylated by reaction with ATP to yield fructose 1,6-bisphosphate. ATP CH2OPO,?- ADP CH2OPO,- 2-OPOCH2 он C=0 B-Fructose 1,6-bisphosphate но но -н CH2OPO, Н HO- Он ОН O Fructose 1,6-bisphosphate undergoes ring opening and is cleaved by a retro-aldol reaction into glyceraldehyde 3-phosphate and dihydroxyacetone phosphate (DHAP). DHAP then isomerizes to glyceraldehyde CH2OPO,- 3-phosphate. но о Гсн-он -0,Росн-снсн 2-O3POCH,ČCH,OH C=0 HO- H- Dihydroxyacetone phosphate Glyceraldehyde 3-phosphate | Cн-ОPО CH2OPO,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
As we saw in Section 291 formation of glucose 6phosphate from glucose and ATP i...View the full answer

Answered By

Anjali Arora
Having the experience of 16 years in providing the best solutions with a proven track record of technical contribution and appreciated for leadership in enhancing team productivity, deliverable quality, and customer satisfaction. Expertise in providing the solution in Computer Science, Management, Accounting, English, Statistics, and Maths.
Also, do website designing and Programming.
Having 7 yrs of Project Management experience.
100% satisfactory answers.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The oven shown in Figure has a heating element with appreciable capacitance C1. The other capacitance is that of the oven air C. The corresponding temperatures are T1 and T2, and the outside...
-
The biosynthetic pathway shown in Figure 26.10 was developed with the aid of isotopic labeling experiments. Which carbon atoms of cholesterol would you expect to be labeled when acetate enriched with...
-
The biosynthetic pathway shown in Figure 26.10 was developed with the aid of isotopic labeling experiments. Which carbon atoms of cholesterol would you expect to be labeled when acetate enriched with...
-
This chapter describes the mechanisms in place to regulate accounting and financial reporting in five countries. Required: Compare and contrast these mechanisms in the United Kingdom and China.
-
What are the differences between GAAP based and Pro Forma financial statements?
-
Let X Gamma(a, ), where a is a large integer. Without doing any calculations, explain why X Norm(a/, a/ 2 ).
-
Recall the WEKA Logistic example for classifying cereals as either high or low. Compute the probability that the fourth instance from the test set is classified either high or low. Does your...
-
Using the Consolidated Statements of Operations and the excerpts from the Logitech International S.A. Form 10-K, analyze the profitability of Logitech. Your analysis should include the following...
-
uiz Instructions ver the following Question 8 1 pts Which of the following approaches can be used to satisfy the equal-service requirement when comparing two alternatives? None of the above Sudy...
-
Consider an option on a stock when the stock price is $41, the strike price is $40, the risk-free rate is 6%, the volatility is 35%, and the time to maturity is 1 year. Assume that a dividend of...
-
What enzyme cofactor is associated with each of the following kinds of reactions? (a) Transamination (b) Carboxylation of a ketone (c) Carboxylation of an -keto acid
-
In the pentose phosphate pathway for degrading sugars, ribulose 5-phosphate is converted to ribose 5-phosphate. Propose a mechanism for theisomerization. CH2 - HO- C=0 HO- - - HO- -OH H. -HO- -...
-
When should a preliminary bid day total be made, and why?
-
A company which manufactures microwaves advertises that 90% of their microwaves are flawless, requiring no adjustments. Their quality control department tests this percentage on a regular basis. On...
-
A new retail store is being planned for a site that contains 40 ft of soft clay (c 0.075 ft2/day, y = 100 pcf). The clay layer is overlain by 15 ft of sand (y = 112 pcf) and is underlain by dense...
-
Perez Bags (PB) is a designer of high-quality backpacks and purses. Each design is made in small batches. Each spring, PB comes out with new designs for the backpack and for the purse. The company...
-
Find a recent (within the last 12 months) article or economic blog related to price fixing, provide an executive summary of the information. Include an APA reference and/or link. How does the fact...
-
A rectangular block of a material with a modulus of rigidity G=90 ksi is bonded to two rigid horizontal plates. The lower plate is fixed, while the upper plate is subjected to a horizontal force P....
-
What is brainstorming, and what are its pros and cons?
-
Listed below are common types of current liabilities, contingencies, and commitments: a. Accounts payable b. Bank loans and commercial paper c. Notes payable d. Dividends payable e. Sales and excise...
-
Redo Problem 14.8 using Aspen Plus. Problem 14.8 Methane is to be burned in air. Determine the adiabatic flame temperature as a function of the methane-to-air ratio at a pressure of 1 bar.
-
The two dimers of 2-methylpropene shown in the equation can be converted to 2,2,4-trimethylpentane (known by its common name isooctane) for use as a gasoline additive. Can you suggest a method for...
-
Write the structure of the major organic product formed in the reaction of 1-pentene with each of the following: (a) Hydrogen chloride (b) Hydrogen bromide (c) Hydrogen bromide in the presence of...
-
Repeat Problem 6.22 for 2-methyl-2-butene. In Problem 6.22 Write the structure of the major organic product formed in the reaction of 1-pentene with each of the following: (a) Hydrogen chloride (b)...
-
The rate of return on Cherry Jalopies, Inc., stock over the last five years was 14 percent, 11 percent, 4 percent, 3 percent, and 7 percent. What is the geometric return for Cherry Jalopies, Inc.?
-
U.S. GAAP specifies all of the following characteristics of variable interest entities except: A. Equity holders hold less than 5% of the entitys voting stock. B. Equity holders do not have voting...
-
Rank the following three stocks by their risk-return relationship, best to worst. Night Ryder has an average return of 10 percent and standard deviation of 27 percent. The average return and standard...

Study smarter with the SolutionInn App


