The hydration of an alkyne is not a reasonable preparative method for each of the following compounds.
Question:
The hydration of an alkyne is not a reasonable preparative method for each of the following compounds. Explain why.
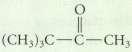
Transcribed Image Text:
(CH)C C CH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 87% (16 reviews)
Hydration can only be used to prepare ketones tha...View the full answer

Answered By

Shem Ongek
I am a professional who has the highest levels of self-motivation. Additionally, I am always angled at ensuring that my clients get the best of the quality work possible within the deadline. Additionally, I write high quality business papers, generate quality feedback with more focus being on the accounting analysis. I additionally have helped various students here in the past with their research papers which made them move from the C grade to an A-grade. You can trust me 100% with your work and for sure I will handle your papers as if it were my assignment. That is the kind of professionalism that I swore to operate within. I think when rating the quality of my work, 98% of the students I work for always come back with more work which therefore makes me to be just the right person to handle your paper.
4.80+
174+ Reviews
426+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Write an equation for the synthesis of 2-octanone by a. Oxidation of an alcohol b. Hydration of an alkyne
-
The standard free energy of activation (G++) for hydration of 2-methylpropene to 2-methyl-2-propanol (Eq. 4.41, p. 169) is 91.3 kJ mol-t (Zt 8 kcal mol-l;. The standard free energy G for hydration of...
-
Hydration of alkynes (via oxymercuration) gives good yields of single compounds only with symmetrical or terminal alkynes. Show what the products would be from hydration of each compound. (a)...
-
Establish procedures to guarantee substantiation of claims for allowances. Think about how you would set up a process to ensure employee claims and allowances could be claimed. Write a step by step...
-
MMC maintains a qualified defined benefit plan for eligible employees, with an effective date of January 1, 1990. The plan year for vesting and participation purposes is the calendar year....
-
Explain how the variablegrowth- rate technique could be used for a firm whose dividend is not expected to grow for three years and then will grow at 5 percent indefinitely.
-
How can software assist in project communications? How can it hurt project communications? LO.1
-
Fred, age 50, plans to retire when he reaches age 65. He is considering investing in either an IRA or a Roth IRA. He plans to contribute $6,000 per year until he retires. Fred expects his marginal...
-
Answer the question below about Not-for-Profit entities using complete sentences and proper references to the authoritative code(FASB Accounting Standards Codification). Explain how the presentation...
-
Rio Ferinand, the owner of Ferdinand Gold Mining, is evaluating a new gold mine in Fort McMurray. Paul Pogba, the company's geologist, has just finished his analysis of the mine site. He has...
-
Draw a Lewis structure for each of the following alkynes. (a) isopropylacetylene (b) cyclononyne (c) 4-methyl-1-pentyne
-
Compare the results of hydroboration-oxidation and mercuric ion-catalyzed hydratioa for 2-butyne.
-
Find a 1 and d for the arithmetic series. S 12 = -108, a 12 = -19
-
Locate a scholarly article relevant to how to present your financial plan for opening a Roller Skating Rink (from your draft business plan) to a lending institution--and describe your strategy for...
-
How would you expect seasonal fluctuations in demand to affect a rental company's decisions about pricing rented products such as wedding dresses or convertible cars? In terms of pricing principles,...
-
Do we drive technology, or does technology drive us? If technology drives us, what are the risks? The other side of the coin would be that we are able to stay ahead of technological transformations....
-
How do you explain the differences between the two analyses and what are the implications of using the BCG matrix in practice?
-
How do leadership styles, such as transformational leadership, shared leadership, and servant leadership, impact team dynamics, member motivation, and overall team effectiveness ?
-
What are the implications of the Miller model under various tax assumptions?
-
What kind of financial pressures can an LBO cause?
-
Answer the following questions for the 2,4,6-heptatrienyl cation. (a) Which MO is nonbonding? (b) Classify each MO as symmetric or antisymmetric. (c) To which carbon atoms in this cation is the...
-
Classify the following sigmatropic reaction by giving its bracketed-number designation and its stereochemistry with respect to the plane of the -electron system. CH3 CH3 H S configuration
-
(a) Explain why two monomethyl esters of N-acetyl-laspartic acid are known. Draw their structures. (b) Explain why a mixture of these two compounds can be separated by cation-exchange chromatography...
-
Pottery Ranch Inc. has been manufacturing its own finials for its curtain rods. The company is currently operating at 100% of capacity, and variable manufacturing overhead is charged to production at...
-
3. How much life insurance do you need? Calculating resources - Part 2 Aa Aa E Paolo and Maria Rossi have completed Step 1 of their needs analysis worksheet and determined that they need $2,323,000...
-
On March 1, LGE asks to extend its past-due $1,200 account payable to Tyson, Tyson agrees to accept $200 cash and a 180-day, 8%, $1,000 note payable to replace the account payable. (Use 360 days a...

Study smarter with the SolutionInn App


