The integrated 1H NMR spectrum of a compound of formula C4H10O is shown in figure. Propose astructure.
Question:
The integrated 1H NMR spectrum of a compound of formula C4H10O is shown in figure. Propose astructure.
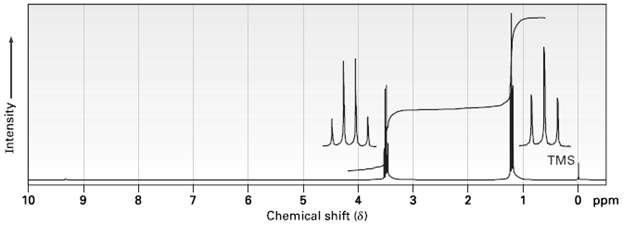
Transcribed Image Text:
TMS 10 8. 6. Chemical shift (8) 3 O ppm Intensity 6.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (8 reviews)
The molecular formula C4H10O indicates that the compound has no ...View the full answer

Answered By

CHARLES AMBILA
I am an experienced tutor with more than 7 years of experience. I have helped thousands of students pursue their academic goals. My primary objective as a tutor is to ensure that students have easy time handling their academic tasks.
5.00+
109+ Reviews
324+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The 1H NMR spectrum of a compound (C10H13BrO) is shown in Figure 16.10. The compound gives benzyl bromide, along with a second compound C3H6Br2, when heated with HBr. What is the first compound?
-
The 1H NMR spectrum of a compound (C10H13BrO) is shown in Figure 16.10. The compound gives benzyl bromide, along with a second compound C3H6Br2, when heated with HBr. What is the first compound?
-
The proton NMR spectrum of a compound of formula C 10 H 12 O follows. This compound reacts with an acidic solution of 2,4-dinitrophenylhydrazine to give a crystalline derivative, but it gives a...
-
From the information below, 1. List of the working capital accounts. 2. Calculate the net working capital. Buildings $100,000 Cash ... 5,000 Trade receivables ... 25,000 Trade and other payables .....
-
The large turbine generator industry is a duopoly. The two firms, GE and Westinghouse, compete through Cournot quantity setting competition. The demand curve for the industry is P=100-Q, where P is...
-
In the SternGerlach experiment, why is it essential for the magnetic field to be inhomogeneous (that is, nonuniform)?
-
Consider the following time series: Period Sales Period Sales Period Sales 1 37 6 29 11 18 2 30 7 24 12 16 3 22 8 17 13 21 4 28 9 22 14 17 5 25 10 24 15 15 a. Use PHStat and set a = 0.05 to test this...
-
Fred, age 50, plans to retire when he reaches age 65. He is considering investing in either an IRA or a Roth IRA. He plans to contribute $6,000 per year until he retires. Fred expects his marginal...
-
wesion
-
Shorecliff College, a liberal arts college located on the West Coast, provides dormitory housing for approximately half of its students. Students who choose not to live on campus, or who do not get...
-
Draw structures for compounds that meet the following descriptions: (a) C2H6O; one singlet (b) C3H7Cl; one doublet and one septet (c) C4H8Cl2O; two triplets (d) C4H8O2, one singlet, one triplet, and...
-
3-Bromo-1-phenyl-1-propene Shows a complex NMR spectrum in which the vinylic proton at C2 is coupled with both the Cl vinylic proton (J = 16 Hz) and the C3 rnethylene protons (J = 8Hz). Draw a tree...
-
Do people have an overconfidence attitude about their financial situation compared to others? Estimate the number of American adults who say that they belong in the upper class (CLASS: 4 = Upper...
-
4. Thinking Ahead (2 points): Project 1 involves the analysis of a bicycle pedal. Consider the bicycle shown below. If a rider places their full weight on the pedal when it is in the horizontal...
-
On January 1, 2023, Martineau Corp. issued a 5-year, 5% installment note payable for $118,000 to finance upgrading its current equipment. The company's year end is December 31. The repayment of...
-
Multiply. 2 x-x-2 3x-3 2 x+2x-3 x+1 Simplify your answer as much as possible.
-
Explain the processes of querying a relational database and define Big Data and explain its basic characteristics. Compare and contrast the major types of networks. - Identify the fundamentals of...
-
42. Explain why the inequality x - x + 1 < 0 has the empty set as the solution set.
-
Describe different sampling methods commonly used by business researchers.
-
Estimate a range for the optimal objective value for the following LPs: (a) Minimize z = 5x1 + 2x2 Subject to X1 - x2 3 2x1 + 3x2 5 X1, x2 0 (b) Maximize z = x1 + 5x2 + 3x3 Subject to X1 + 2x2 +...
-
An ethylene glycol solution contains 21.2 g of ethylene glycol (C 2 H6O 2 ) in 85.4 mL of water. Determine the freezing point and boiling point of the solution.
-
For each spectrum, interpret all the significant stretching frequencies above 1580 cm-1. wavelength (um) 2.5 100 4 4.5 9 10 12 13 14 15 16 60 N 40 T 1642 4000 3500 3000 2500 2000 1800 1600 1400 1200...
-
Point out which of these four mass spectra indicate the presence of sulfur, chlorine, bromine, iodine, or nitrogen. Suggest a molecular formula for each? 100 156 158 80 40 20 0 10 2030 40 50 60 70 80...
-
Show the fragmentation that accounts for the cation at m/z 57 in the mass spectrum of 2-methylpentane. Explain why this ion is less abundant than those at m/z 71 and 43?
-
3 . Accounting.. How does depreciation impact financial statements, and what are the different methods of depreciation?
-
NEED THIS EXCEL TABLE ASAP PLEASE!!!! Presupuesto Operacional y C lculo del COGS Ventas Proyectadas: Ventas Proyectadas: $ 4 5 0 , 0 0 0 Precio por unidad: $ 4 5 0 Unidades vendidas: 4 5 0 , 0 0 0 4...
-
The wash sale rules apply to disallow a loss on a sale of securities_______? Only when the taxpayer acquires substantially identical securities within 30 days before the sale Only when the taxpayer...

Study smarter with the SolutionInn App


