The O ? H hydrogen in acetic acid is much more acidic than any of the C
Question:
The O ? H hydrogen in acetic acid is much more acidic than any of the C ? H hydrogens. Explain this result using resonance structures.
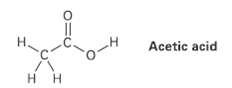
Transcribed Image Text:
Acetic acid
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
H 0 HC 0 HH 0 HH The OH hydrogen of acetic acid i...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The pKa of the starting material (Ar-OH) is ~9, which is much more acidic than a normal alkyl alcohol (pKa ~15)? The reason is resonance stabilization of the conjugate base. Please draw the...
-
Pyrrole is much more acidic than diisopropylamine. Explain. pka = 16 pKa %3D pka = 36 Z-I Z-
-
One of the following hydrocarbons is much more acidic than the others. Indicate which one, and explain why it is unusually acidic.
-
A business has the following transactions: The business is started by receiving cash from an investor in exchange for common stock $20,000 The business purchases supplies on account $500 The...
-
Sally owns real property for which the annual property taxes are $9,000. She sells the property to Shelley on March 1 for $550,000. Shelley pays the real property taxes for the entire year on October...
-
Solve the given differential equation by using an appropriate substitution. dy/dx = (1 - x y)/( x + y)
-
Use the PewWorkPlay dataset to address this question: do the populations of men and women differ in how they feel about online dating, as measured by scores on the Online Dating Attitude Index?
-
The following trial balance was taken from the records of Fairport Manufacturing Company at the beginning of 2019: Transactions for the Accounting Period 1. Fairport purchased $11,400 of direct raw...
-
Penny Lane bonds have a 7.5% annual coupon rate and pay interest semi-annually. The face value is $1,000 and the current market price is $1,010.00 per bond. The bonds mature in 7 years. The current...
-
Each of the following languages is the intersection of two simpler languages. In each part, construct DFAs for the simpler languages, then combine them using the construction discussed in footnote 3...
-
Alcohols can act either as weak acid or as weak bases, just as water can. Show the reaction of methanol, CH 3 OH, with a strong acid as HC1 and with a strong base such as NA + - NH 2 .
-
Write the products of the following acid-base reactions: (a) CH3OH + H2SO4 ? (b) CH3OH + NaNH2 ? (c) CH3NH3 + C1- +NAOH ?
-
Jane Myers is the manager of an extremely successful gift shop, Janes Gifts, which is operated for the benefit of local charities. From the data below, she wants a cash budget showing expected cash...
-
Small paragraphs address these sub questions, 1. What is governance? 2. What drives the strategy orientation of the leadership ? 3. How do strategies trickle down to management and translate to...
-
Accommodating guests with disabilities must be a priority for venue and event managers. With a growing population of persons with disabilities, it's important to identify current trends on how venues...
-
a. (6) The Fibonacci sequence is defined by Fo= 0, F = 1, and, for all n > 2, Fn = Fn-1+ Fn-2- Prove that Fn O(2"), without using part (b). b. (6) Let An C {0, 1}" be the set of binary strings of...
-
Customer purchase history matrix. A store keeps track of its sales of products from K different product categories to N customers over some time period, like one month. (While it doesn't matter for...
-
2. A Beautiful Circuit Answer the following questions about the 4-resistor circuit shown below. A. Calculate the equivalent resistance of this circuit. B. Calculate the power delivered to the circuit...
-
Which paragraph is most appropriate? a. The concept of appraising staff must attach to some form of professional foundation for it to have any real meaning. If it is not seen as part of a career...
-
Read the following description and Write a response of it. The discretion of public administrators can be decreased, but not altogether eliminated. Officials will use their discretion in any given...
-
Steam is produced at 70 bar and some unknown temperature. A small amount of steam is bled off just before entering a turbine and goes through an adiabatic throttling valve to atmospheric pressure....
-
Indicate whether each of these objects is chiral or achiral: (a) Golf ball (b) Baseball glove (c) Clock (d) T-shirt (e) Dress shirt (f) Automobile
-
Determine whether each of these molecules is chiral, for those that are chiral, put an asterisk at the chirality center. b) a) d) I e) )
-
Indicate whether each of these objects or molecules has a plane of symmetry: c) Ear b) Pencil a) Idealized human face I CH3 e) f) d) CH3 . Cl Br CH3 "H. CH3 "H g) h) Cl
-
Eye Deal Optometry leased vision - testing equipment from Insight Machines on January 1 , 2 0 2 4 . Insight Machines manufactured the equipment at a cost of $ 2 0 0 , 0 0 0 and lists a cash selling...
-
help! ee all photos + Add to o e D C N X Edit & Create Share Table of Contents No sales to an individual customer accounted for more than 10% of revenue during any of the last three fiscal years. Net...
-
Business law A person may have the liability of a partner even though no partnership exists True False

Study smarter with the SolutionInn App


