The oral contraceptive agent Mestranol is synthesized using a carbonyl addition reaction like that shown in Problem
Question:
The oral contraceptive agent Mestranol is synthesized using a carbonyl addition reaction like that shown in Problem 8.42. Draw the structure of the ketoneneeded.
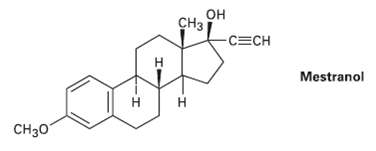
Transcribed Image Text:
он CH3 CECH н Mestranol Н CH30
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (15 reviews)
CH3O CH3 1 ...View the full answer

Answered By

Jayshree Rathi
Hello Students!
This is Jayshree Rathi. I work on a number of renowned student-centric channels such as Chegg, coursehero, as a certified private tutor.
If you are looking for relevant and original content to complete your assignments, essays, and homework, then contact me and within the promised time, I will deliver you your personalized academic work and help you score the best.
4.80+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
A possible alternative mechanism to that shown in Problem 24 for the monochlorination of ethane would involve the following propagation steps: CH3--H + Cl - CH3--Cl + H H + Cl--Cl - H--Cl + Cl How do...
-
A charged line like that shown in Fig. 21.25 extends from y = 2.50 cm to y = - 2.50 cm. The total charge distributed uniformly along the line is -9.00 nC. (a) Find the electric field (magnitude and...
-
A conductor with an inner cavity, like that shown in Fig. 22.23c, carries a total charge of + 5.00 nC. The charge within the cavity, insulated from the conductor, is -6.00 nC. How much charge is on...
-
The three courses below represent a polygon. They are consecutive azimuths measured clockwise from North in DMS. Course A to B: 55-35-21 Course B to C: 175-48-19 Course C to A: 293-22-28 Choose the...
-
The Andrews Apple Products Company purchases apples from local growers and makes applesauce and apple juice. It costs $0.80 to produce a jar of applesauce and $0.60 to produce a bottle of apple...
-
A cross-flow molecular filtration device equipped with a mesoporous membrane is used to separate the enzyme lysozyme from a fermentation broth, as shown in the figure (right column). Water at...
-
FNBC is a business brokerage firm that assits in the purchase and sale of businesses. Jennings and Hennessey were independent contractors working for FNBC. They left FNBC, and FNBC sued them for...
-
Forgetta Manufacturing has old equipment that cost $48,000. The equipment has accumulated depreciation of $28,000 and a fair value of $26,000. Forgetta has decided to sell the equipment. (a) What...
-
On January 1 , 2 0 2 5 , Joiner, Inc. decides to invest in 9 , 4 5 0 shares of Gelding stock when the stock is selling for $ 1 8 per share. On June 1 , 2 0 2 5 , Gelding paid a $ 1 . 1 0 per share...
-
On January 1, 2014, Parker, Inc., a U.S.-based firm, acquired 100 percent of Suffolk PLC located in Great Britain for consideration paid of 52,000,000 British pounds (), which was equal to fair...
-
Organo metallic reagents such as sodium acetylide undergo an addition reaction with ketones, giving alcohols: How might you use this reaction to prepare 2-methyl-1, 3-butadiene, the starting material...
-
Erythrogenic acid, C 18 H 26 O 2 , is an acetylenic fatty acid that turns a vivid red on exposure to light. On catalytic hydrogenation over a palladium catalyst, 5 equivalents of H 2 is absorbed, and...
-
What are the general guidelines used to determine where your business must pay sales taxes for sales made online?
-
Define subjective brightness and brightness adaptation?
-
Write Down The Properties Of Haar Transform?
-
Explain Spatial Filtering?
-
What Is Maximum Filter And Minimum Filter?
-
Name The Categories Of Image Enhancement And Explain?
-
Special events differ from daily events. They occur spontaneously, invite expectations, are planned, and recognize a unique moment in time with ceremony and ritual to satisfy specific needs. LO.1
-
The Home Depot is the leading retailer in the home improvement industry and one of the 10largest retailers in the United States. The company included the following on its January 29, 2012, balance...
-
What are oxidation states?
-
For each of these ions, draw the important resonance forms and predict which resonance form is likely to be the major contributor. (a) (b) (c) CH2 CH CH2 HN
-
Give a definition and an example for each class of organic compounds. (a) Alkane (b) Alkene (c) Alkyne (d) Alcohol (e) Ether (f) Ketone (g) Aldehyde (h) Aromatic hydrocarbon (i) Carboxylic acid (j)...
-
Cyclopropane (C3H6, a three-membered ring) is more reactive than most other cycloalkanes. (a) Draw a Lewis structure for cyclopropane. (b) Compare the bond angles of the carbon atoms in cyclopropane...
-
The Work in Process inventory account of a manufacturing company shows a balance of $2,400 at the end of an accounting period. The job cost sheets of the two uncompleted jobs show charges of $400 and...
-
1. An investor buys a three-year bond with a 5% coupon rate paid annually. The bond, with a yield-tomaturity of 3%, is purchased at a price of 105.657223 per 100 of par value. Assuming a 5bp change...
-
Describe how the following affect the valuation of PPE. a) Cash Discounts b) Deferred Payment Contracts

Study smarter with the SolutionInn App


