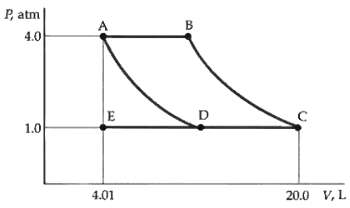
The PV diagram in figure represents 3 mol of an ideal monatomic gas. The gas is initially
Question:
The PV diagram in figure represents 3 mol of an ideal monatomic gas. The gas is initially at point A. The paths AD and BC represent isothermal changes. If the system is brought to point C along the path AEC, find
(a) The initial and final temperatures,
(b) The work done by the gas, and
(c) The heat absorbed by the gas.
Transcribed Image Text:
P, atm 4.0 1.0 20.0 V, L 4.01
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
Although not required for this problem we begin by de...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Fundamentals of Ethics for Scientists and Engineers
ISBN: 978-0195134889
1st Edition
Authors: Edmund G. Seebauer, Robert L. Barry
Question Posted:
Students also viewed these Thermodynamics questions
-
In a refrigerator, 2.00 mol of an ideal monatomic gas are taken through the cycle shown in the figure. The temperature at point A is 800.0 K. (a) What are the temperature and pressure at point D? (b)...
-
A 2.0 mol sample of an ideal monatomic gas undergoe the reversible process shown in Figure, the scale of the vertical axis is set by Ts = 400.0K and the scale of the horizontal axis is set by Ss =...
-
The temperature of 2.00 mol of an ideal monatomic gas is raised 15.0 K at constant volume. What are (a) The work W done by the gas, (b) The energy transferred as heat Q, (c) The change Eint in the...
-
In Exercises explain why Rolle's Theorem does not apply to the function even though there exist a and b such that (a) = (b). f(x) H [1,1]
-
Vitex, Inc. manufactures a popular consumer product and it has provided the following data excerpts from its standard cost system: The company's manufacturing overhead cost is applied to production...
-
The Insurance Institute for Highway Safety conducted tests with crashes of new cars traveling at 6 mi/h. The total cost of the damages was found for a simple random sample of the tested cars and...
-
If you were to follow up the Slonaker and Wendt (2003) study on discrimination against African American males, what philosophical stance may underpin your research choice? LO6
-
From the following information, draw the project network. Compute the early, late, and slack times for each activity. Identify the critical path. (Draw the finish-to-start relationshipsfirst.)...
-
Q1 a.Discuss why organizations are concern about security (and reliability) of their information systems. b.What are the three main sources of threats to the information security of an organisation?...
-
CableTech Bell Corporation (CTB) operates in the telecommunications industry. CTB has two divisions: the Phone Division and the Cable Service Division. The Phone Division manufactures telephones in...
-
What is the number of moles n of the gas in Problem79? P, atm A 4.0 1.0 4.01 20.0 V, L
-
Repeat Problem 81 with the gas following path ABC. P, atm 4.0 1.0 20.0 V, L 4.01
-
Use an appropriate series in (2) to find the Maclaurin series of the given function. Write your answer in summation notation. xe 3x
-
Find the unknown angle measures. 49 60 Drawing is not to scale. I = y = In S
-
Q5 For this question, use data from only restaurants with between 50 and 60 items in the data set. Predict total fat from cholesterol, total carbs, vitamin a, and restaurant. Remove any...
-
A meteorologist believes that there is a relationship between the daily mean windspeed, w kn, and the daily mean temperature, t C. A random sample of 9 consecutive days is taken from past records...
-
Suppose k(x) = f(g(h(x))). Given the table of values below, determine k' (1). g(x) h(x) f'(x) g'(x) h'(x) x f(x) 1 -6 -3 3 6 -6 -6 3 -3 4 1 -7 -2 5 4 -2 7 3 1 -7 -8
-
In a research study women with metastatic stomach cancer responded to the Symptom Distress Scale and the Profile of Mood States. A correlation coefficient was reported: r = 0.5, p = 0.03. How would...
-
Give two reasons why most organizations use a six-month or annual period rather than a weekly or monthly period to compute budgeted indirect-cost rates.
-
Figure displays a 12.0 V battery 3 four uncharged capacitors of capacitances C1 = 4.00F, C2 = 6.00F, and C3 = 3.00F. The switch is thrown to the left side until capacitor 1 is fully charged. Then the...
-
How does the power of a dry contact lens compare with its power when resting on the tear layer of the eye? Explain.
-
In Example 21.3, calculate the net force on charge q1.
-
In Example 21.4, what is the net force (magnitude and direction) on charge q1 exerted by the other two charges?
-
Three point charges are arranged along the x-axis. Charge q1 = +3.00C is at the origin, and charge q2 = -5.00C is at x = 0.200 m. Charge q, = - 8.00 C. Where is q3 located if the net force on q1 is...
-
Question 24 Miami Company sold merchandise for which it received $710,400, including sales and excise taxes. All of the firms sales are subject to a 6% sales tax but only 50% of sales are subject to...
-
f the IRS intends to close a Taxpayer Assistance Center, they must notify the public at least _____ days in advance of the closure date. 14 30 60 90
-
A company is evaluating a new 4-year project. The equipment necessary for the project will cost $3,300,000 and can be sold for $650,000 at the end of the project. The asset is in the 5-year MACRS...

Study smarter with the SolutionInn App


